The National Institute for Health and Care Excellence (NICE) has recommended against the use of Gilead’s Yescarta (axicabtagene ciloleucel) on the National Health Service (NHS) network.
The decision comes a day after Yescarta secured marketing authorisation from the EU to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This authorisation allows marketing of the drug in 28 EU countries, including the UK, along with Norway, Iceland and Liechtenstein. In England, the drug is made available by Gilead unit Kite Pharma.
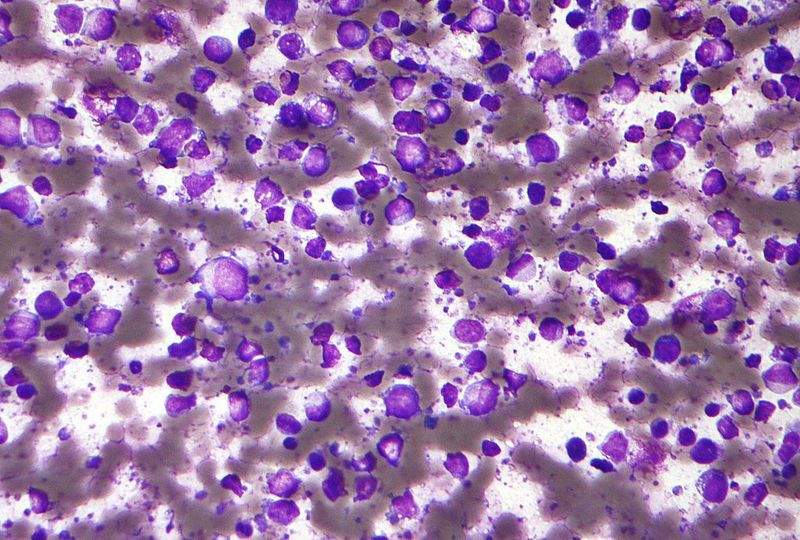
Axicabtagene ciloleucel is a chimeric antigen receptor T-cell (CAR-T) therapy designed to leverage the patient’s own immune system against some types of blood cancer. It is specifically manufactured for individual patients.
A NICE committee found that the cost-effectiveness estimates for Gilead’s Yescarta were more than £50,000 a year of quality-adjusted life (QALY) gained, when compared to salvage chemotherapy.
Based on the findings, the committee concluded that the cost of the CAR-T therapy is very high for it to be considered as a cost-effective use of NHS resources.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataNICE added that axicabtagene ciloleucel lacks direct data for comparison with the current standard, thereby limiting the information on the exact size of its benefit compared with salvage chemotherapy.
NICE Centre for Health Technology Evaluation director Meindert Boysen said: “Although promising, there is still much more we need to know about CAR-T, and unfortunately, in this case, we are not able to recommend axicabtagene ciloleucel for use in the NHS in England at the cost per patient set by Kite Pharma.”
Meanwhile, NICE has invited suggestions and further evidence on a new treatment for DLBCL and PMBCL that could lead to its availability on the NHS.




