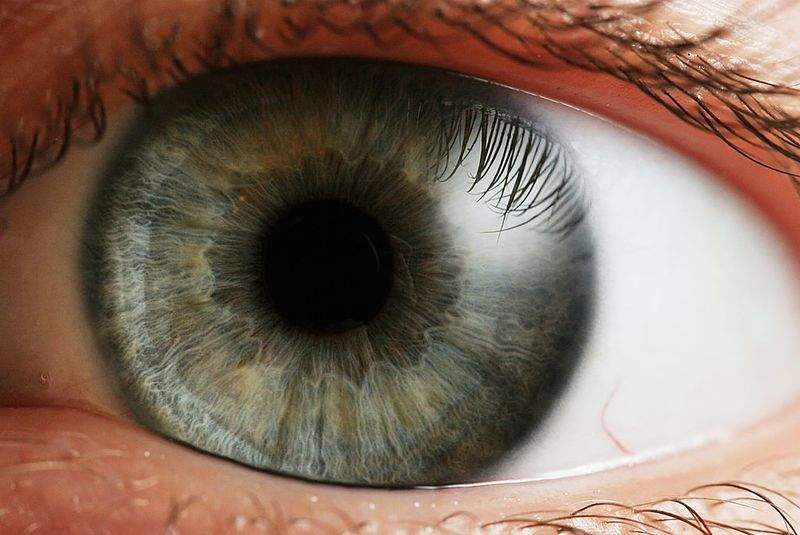
US-based biotechnology firm Wave Life Sciences has revealed plans to develop new therapeutics for the potential treatment of various rare, genetic eye diseases.
The company plans to design and advance stereopure oligonucleotide therapeutics. Initially, the ophthalmology research will focus on four inherited retinal conditions that result in progressive vision loss.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
These diseases are retinitis pigmentosa caused by a P23H mutation in the RHO gene, Stargardt disease, Usher syndrome type 2A, and Leber congenital amaurosis 10.
Wave Life Sciences will explore RHO P23H, USH2A, ABCA4 and CEP290 as treatment targets for these diseases.
The company expects to announce its first development candidate in the second half of next year.
Wave Life Sciences president and CEO Paul Bolno said: “We have long believed that oligonucleotides have the potential to be particularly effective and durable in the eye and are energised by our latest research that provides additional validation of our precisely designed stereopure oligonucleotides.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Our aim is to move quickly to develop long-acting, intravitreally injected, disease-modifying therapies to address the enormous need across a spectrum of rare, genetically-defined eye diseases.”
The firm’s ophthalmology project builds on its non-human data presented at the 14th Annual Meeting of the Oligonucleotide Therapeutics Society held in Seattle, Washington.
Data showed that a single intravitreal stereopure oligonucleotide injection in the eye of non-human primates led to more than 95% knockdown of a target RNA in the retina for at least four months.
Based on this data, Wave Life Sciences aims to design medications that could deliver a therapeutic effect with only two doses per year.
Inherited retinal dystrophies are known to affect approximately 200,000 people in the US. The company anticipates that its new approach will help treat nearly 10,000 patients with the four retinal diseases.


