Amicus Therapeutics has received approval from the US Food and Drug Administration (FDA) for a two-component therapy, Pombiliti (cipaglucosidase alfa-atga) + Opfolda (miglustat) 65mg capsules, to treat Pompe disease.
Pombiliti treats adult patients with late-onset Pompe disease (LOPD) weighing 40kg or under who are not able to improve on their present enzyme replacement therapy (ERT).
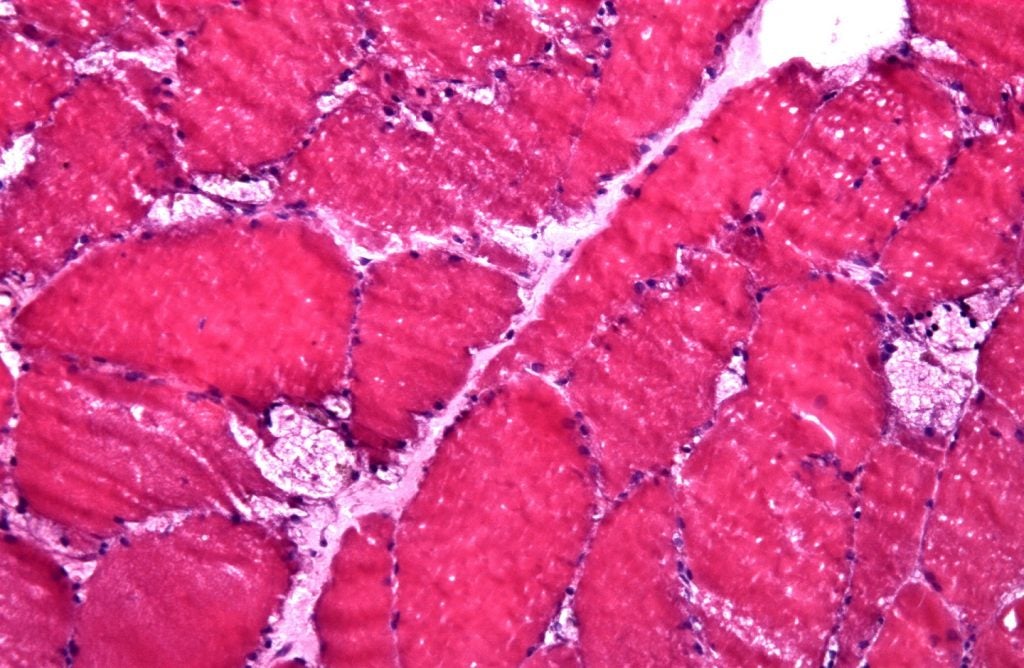
LOPD is a lysosomal disorder caused by a deficiency of the enzyme acid alpha-glucosidase (GAA).
Designed for enhanced uptake into muscle cells, pombiliti is a recombinant human GAA enzyme (rhGAA) naturally expressed with high levels of Mannose 6-Phosphate (bis-M6P).
Upon entering the cell, it will be processed into the active and mature form to break down glycogen.
Opfolda is used to stabilise the enzyme in the blood.
The FDA has approved the therapy based on clinical data from the Phase III PROPEL pivotal study. It is the only LOPD trial to assess ERT-experienced participants in a controlled setting.
Amicus Therapeutics president and CEO Bradley Campbell stated: “Today’s approval is also a testament to Team Amicus’ extraordinary dedication to patients and our ability to execute on our vision to bring new therapies to the rare disease community.
“Our highly experienced team is ready to launch this medicine in the US and we look forward to rapidly bringing this new treatment regimen to all eligible adults living with late-onset Pompe disease who are not improving on their current ERT.”