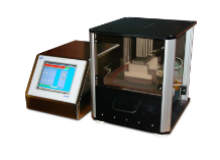
E-Scan 655


The E-Scan 655 uses a high-voltage leak detection (HVLD) technology to inspect vials, syringes, and other liquid-filled parenteral products for container closure integrity.
Using MicroCurrent conductivity test method HVLDmc, the E-Scan 655 does not damage the tested container or product. It even exposes them to lower voltages than other conductivity-based solutions.
This technology uses a non-invasive test method, which requires no sample preparation. E-Scan 655 can be used with a wide range of liquid-based products, including proteinaceous products with suspensions and low-conductivity sterile water for injection (WFI).
The E-Scan 655 features a fast-test cycle and simple to operate. Further benefits include a quick changeover time and easy recipe setup to accommodate a wide range of products and applications.