Extractables and Leachables

Leachables and extractables studies mainly concern materials that are a part of a manufacturing equipment or a packaging.
QualiMextriX’s general strategy fully adopts the Product Quality Research Institute’s (PQRI) documents and the new US Pharmacopeia (USP) monographs (1663 and 1664).
Key features include:
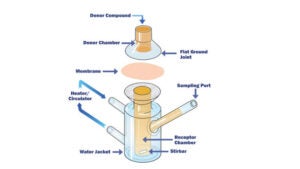
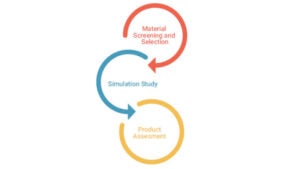
• The applied generic methodology has been designed to cover representative leachables based on designated extraction studies of packaging materials available and are commonly used in plastic manufacturing. This generic methodology is mainly applied to initial extraction studies performed to characterise packaging materials and determine the total available pool of potential leachables.The purpose of the initial screening of extractables is mainly to establish worst-case potential leachables profile for the product-specific packaging materials and facilitate the establishment of quantitative leachable-extractables correlations.
• Based on results obtained from the extraction study, the generic methodology (comprised of the sample pre-treatment and analysis stages) is properly adjusted in order to become a tailor-made product-specific methodology targeting only the analytes / potential leachables species, identified during the extraction study that exceed or have the potential to exceed the products’ analytical evaluation threshold (AET) during the actual leachables study.
The next step is to make this tailor-made method also fit-for-purpose by means of method validation according to ICH Q2.
• The validated product-specific methodology is subsequently applied in order to perform the actual leachable testing and provide reliable quantitative results for the leachables of interest.
In case a compound exceeds the AET the following action plan is implemented:
• The compound’s structure is elucidated to an extent that literature and structure-activity relationship assessment can be performed.
• A literature search through all available databases is performed in order to gather all information necessary for the assessment. A specific absorption rates (SAR) evaluation is performed in-silico by the use of available software such as DEREK in order to be in line with the M7 guideline (ruled-based and statistical-based methodology).
• In case the assessment results in safety concerns with respect to potential mutagenic toxic effects, a risk-based approach is applied based on the available data to evaluate the safety impact by considering the patient population and the duration of use.
• In case the risk exceeds the acceptable level an in-vitro bacterial reverse mutation test is performed as a minimum screen for the assessment of the compound’s genotoxic potential.
The laboratory possesses a cutting edge high-resolution mass spectrometry (HRMS) instrumentation by Thermo Scientific, Orbitrap Elite. It is a hybrid ion trap-orbitrap mass spectrometer with very high resolving power, high-speed, sensitivity and advanced fragmentation information for the non-volatile compounds.
Powerful software is employed for the detection, identification and structural elucidation of analytes.
Gas chromatography (GS) combined with a quadrupole mass analyser (GC-MS) is applied for the determination of volatile and semi-volatile compounds. An electron ionisation source is employed (EI) in order to achieve the fragmentation of the eluted compounds producing characteristic patterns used for the tentative identification of analytes by National Institute of Standards and Technology (NIST) similarity matching. Substitution to a headspace autosampler unit allows for the profiling of highly volatile species.
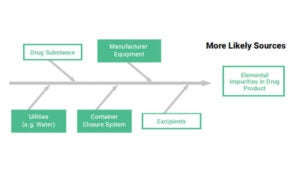
ICP-MS is employed for the detection of traces of elemental impurities in order to cover all range of compounds.