VeriPac 455
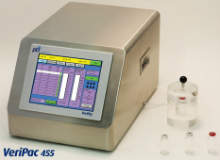

VeriPac 455, a non-destructive inspection system, is suitable for highly sensitive micro leak detection.
The system can be used for empty and pre-filled syringes, liquid filled and lyophilised vials and other flexible and rigid liquid-filled packaging.
VeriPac 455 can be incorporated into protocols in the handling process as it is not destructive or invasive, and requires no sample preparation.
Applications include stability and clinical trial studies, quality assurance testing and production statistical process control (SPC).
The system can detect as little as 0.05 cc/min. Results are more reliable than the dye ingress test.
VeriPac 455’s technology is based on the ASTM vacuum decay leak test method (F2338-09) accredited by the FDA for package integrity testing.
VeriPac 455 features dual vacuum transducer technology, PERMA-Vac that provides increased test sensitivity and produces reliable results.
The system also features advances in internet connectivity and networking capabilities that facilitate remote operation, system monitoring and troubleshooting.
In addition, VeriPac 455 supports sustainable packaging initiatives due to no waste.
VeriPac 455 connects to a test chamber that is designed to contain the package. Dual transducer technology monitors the test chamber for the level and the change in vacuum over a predetermined test time.
Changes in absolute and differential vacuum indicate leaks and defects within the package. Test systems are for manual or automatic operation. This inspection method is suitable for laboratory offline testing and QA/QC statistical process control. Cycles take a few seconds.
VeriPac 455 measures seal integrity of entire container or package and verifies container closure system integrity. It tests for gas leaks for dry products such as lyophilised vials, and liquid leaks, including liquid filled vials, pre-filled syringes.