Agendia, a genomics cancer diagnostics company, opened a new clinical genomics laboratory on 5 August 2010. Located in Irvine California, the new laboratory was inaugurated in the presence of politicians in California and other high profile people including Sukhee Kang, the Mayor of Irvine, clinicians and representatives of patient advocacy organisations.
The new laboratory is designed with larger capacity, suited to facilitate Agendia’s commercial expansion in the US. Agendia earlier had its offices and genomics laboratory housed in a facility located in Huntington Beach, California, that was opened in May 2009. The 8,000ft² facility was licensed and registered by the Clinical Laboratory Improvements Amendments (CLIA).
Facility
The new facility accommodates corporate offices and a state-of-the-art genomics laboratory that spreads over 15,000ft². The facility was formerly a building with three laboratories and two clean rooms. It was upgraded to include the different laboratory requirements of Agendia and the necessary electrical, plumbing, casework and mechanical works. New electrical labelling was installed while many electrical branch circuits were reworked. Additional equipment included new laboratory sinks, and a new emergency shower and eyewash combination.
Production
The new laboratory will provide increased capacity to meet the rising demand for MammaPrint, a breast cancer recurrence test launched by Agendia in 2004. MammaPrint is the first and only such test that has been approved by the US FDA. The test helps in identifying early metastasis risk among patients and provides a clear rationale to evaluate the benefits of chemotherapy besides other clinical information and pathology tests.
The MammaPrint is currently playing a key role in the I-SPY 2 trial, a breast cancer trial launched by Biomakers consortium in March 2010. The consortium includes the FDA, the National Institutes of Health and other big pharmaceutical companies led by the Foundation for the National Institutes of Health. Designed to use Agendia’s MammaPrint to measure the biomarkers, the trial is being taken by patients at 20 leading cancer research centres in the US.
Technology
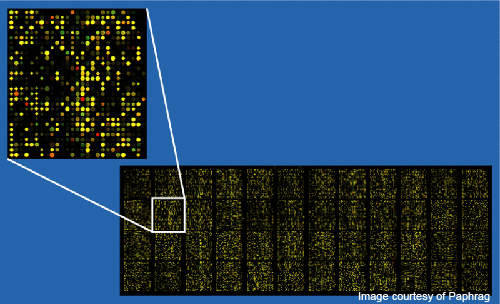
The new laboratory will conduct MammaPrint using microarray analysis technology. The technology uses DNA microarrays, a group of several thousands of genes that are printed over a glass slide. A unique DNA fragment with a specific sequence is found on each spot on the slide. The technology allows scientists to view several genes simultaneously.
The MammaPrint is conducted on a tumor sample after it is surgically removed. Customised microarrays developed by Agilent that comprise a number of carefully selected normalisation genes are used for this test.
Each microarray has three identical sets of the 70-gene profile of the tumor sample that needs to be tested. To assess the activity of the 70 specific genes, the messenger RNA (mRNA) from the sample is removed and labelled with a fluorescent dye. The labeled mRNA is hybridised to the DNA microarray along with a labelled mRNA from a suggested sample.
Scanners are used to detect the fluorescence and establish a digital image that reflects spots on the microarrays, showing where the sampled DNAs have merged. In order to compare gene expression profiles from several patients, the individual chip intensities are normalised to a common standard.
At the end of the microarray process, a score stratifies patients into two groups – patients that are at a lower risk of getting cancer spread to the other parts of the body and others that are at a higher risk.
The score is produced after all the key molecular pathways involved in the metastatic cascade of breast cancer are examined during the MammaPrint test.
Contractors
The contract for the facility construction was awarded to LCS Constructors. They remodelled the building and provided a range of services including electrical, plumbing, and mechanical work.