AstraZeneca France’s Dunkirk plant has been sold to Minafin, a subsidiary of the Minakem Group. The acquisition was completed by 13 May 2009. The facility had been upgraded and expanded for the manufacture of Symbicort, AstraZeneca’s blockbuster asthma and chronic obstructive pulmonary disease drug. AstraZeneca will continue to source specific APIs from the facility as part of a contract-manufacture agreement with Minafin.
The plant was first built in 1991 at a cost of $550m. AstraZeneca France announced a major expansion of its pharmaceutical production facilities in May 2004; this construction was completed in February 2005 and validation followed in August of the same year. The expansion cost an estimated €90m and was supported by grant aid from regional authorities in Normandy, including IFA and Nord France Experts.
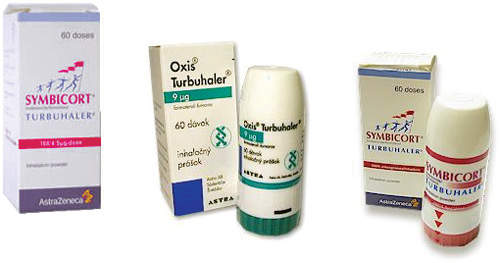
Symbicort generated worldwide sales of more than $549m in 2003. The drug was originally dispensed using a dry powder inhaler – called the Turbuhaler – but since July 2006, has used a pressurised Metered Dose Inhaler (pMDI).
The Dunkirk facility was upgraded for two reasons: to increase the quantity of Symbicort that could be produced at the plant and to begin fill and finish operations for the pMDI at. The pMDI treatment was filed for approval with the European regulatory authorities in July 2004. Another 150 manufacturing personnel were employed following the upgrade, bringing the total workforce to 780.
New aerosol production line
Jacobs Engineering Group of Pasadena, US, won the contract to provide engineering, procurement, construction (EPC), project management and validation for the $25m aerosol production line. The project was completed on a fast-track basis to accommodate the increased global demand for Symbicort in 2005.
A 900m² cleanroom (Class 100,000) facility at the plant was constructed by Carmetec to house the fill and finish section of the production line. Carmatec, based in Uppsala, Sweden completed construction and validation of the cleanroom in 2005; fill and finish manufacturing equipment was installed shortly after.
Symbicort and metered dose delivery
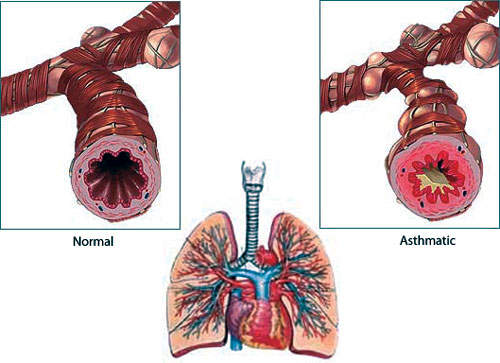
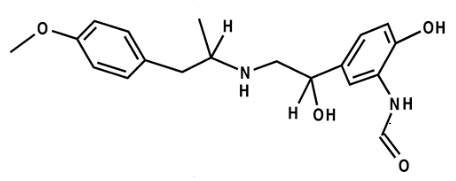
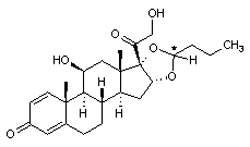
Symbicort is a combination therapy containing budesonide (a corticosteroid) and bronchodilator formoterol in a single inhaler therapy. The drug is indicated in the regular treatment of asthma and chronic obstructive pulmonary disorder, where the combination of an inhaled glucocorticosteroid and a long-acting beta-agonist is appropriate.
The product was originally available as the Symbicort Turbuhaler in standard dose (160/4.5µg) and low-dose (80/4.5µg) strengths. It is approved for use in 90 countries and has been launched in 70. The drug was made available in the new pressurised Metered Dose Inhaler (pMDI) in 2006 to suit new patient preferences.
The system offers easily adjustable dosing, which enables doctors to tailor a patient’s treatment with a single inhaler for all situations, thereby achieving greater efficacy than with fixed doses.
The new generation of respiratory pMDIs use hydrofluorocarbon propellants, which are more environmentally friendly than chlorofluorocarbon propellants and do not damage or deplete the ozone layer. pMDIs have an advantage over the Turbuhaler in that the delivery of medication is not breath-dependent – the dose may be controlled according to requirement.
In January 2005 results from the STAY (US) trial were published in the American Journal of Respiratory and Critical Care Medicine. Data showed that Symbicort Single Inhaler Therapy achieved a 45% reduction in the frequency of severe asthma attacks.
In Europe, in November 2004, the regulatory application for Symbicort Single Inhaler Therapy was withdrawn to allow more data to be submitted. The revised regulatory filing was submitted in the summer of 2005, containing additional data from further ongoing studies, including 13,000 patients with mild to moderate asthma.