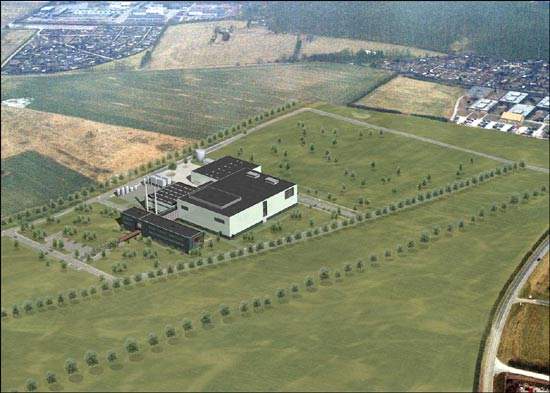
In March 2003 Biogen Idec began construction of its first production facility to be located outside of the USA. The plant, situated 25 miles north of Copenhagen on a 60 acre site at Hillerød, is in an area known as ‘Medicon Valley’ because of its attractiveness to pharmaceutical companies.
The facility is intended to supply the European market and provide back up to the existing US production facilities. The first stage of the facility’s construction has been completed. It included a labelling and packaging facility, an administrative building and laboratory facility apart from equipment installation and partial completion of a bulk manufacturing facility.
The second phase, which involves the construction of a bulk manufacturing facility for the production of TYSABRI, is due for completion in 2011. The capacity requirement of TYSABRI has, however, declined due to improvements in its production. Subsequently, Biogen Idec is considering several options, including delaying the completion of the facility.
.
In late 2003 the plant construction was halted due to restructuring in Biogen Idec following the merger process.
Construction stopped
Prior to the cessation of construction activity the plant was being constructed by the US engineering company Fluor, with the assistance of Novo Nordisk Engineering, Denmark.
The construction was expected to be completed in 2005 and was the first part of a two-stage scheme expected to cost in the region of $350m. Biogen Idec had already secured an option to buy an adjacent 36 acre site for the second phase of construction at Hillerød. It was reported that Biogen Idec was paying for the new facility in cash and had requested no location incentives from the Danish government.
Construction restarted
Jacobs Engineering Group Inc, based in the UK, received a contract from Biogen Idec in May 2007 to provide engineering, procurement services for major process equipment, validation, mechanical completion and site support services for the first cell culture manufacturing facility at the greenfield biotechnology plant in Hillerød, Denmark. The manufacturing facility is expected to produce TYSABRI (natalizumab), which is used in the treatment of multiple sclerosis.
Jacobs had already performed conceptual design and detailed engineering for the facility and will execute this new scope of work from their office in Reading, UK. Jacobs Engineering Group has been linked with the large scale facility at Hillerød since 2005 when work was due to restart.
Biogen Idec had already invested $339m in the plant and was looking to makes changes and expand the partially completed facility to become their leading European biotech facility. A further $225m was invested in 2006.
Plant expansion
The plant expansion requirements are expected to cost in the region of $225m (DKK1.3bn). In December 2006 it was reported that NNE had been assigned to complete the plant. NNE will be in charge of construction management in conjunction with Fluor and also serve as an adviser during the final construction phase of the biotech project. As part of the assignment NNE installed the process equipment in the main manufacturing building which has already been completed in the Hillerød area in the northern region of the island of Zealand. The NNE contract was worth $17.5m (DKK100m).
Paul Coleman, General Manager of the Biogen Idec facility in Hillerød, said: “We are very pleased to form partnership with NNE for the completion of this significant element of our facility in Hillerød. We have worked with the NNE organisation on a previous part of the site expansion and we have been impressed by their expertise, commitment and approach to partnering towards a successful outcome for all parties.” Depending upon the demands of the market a further $100m may be invested in a future production expansion at the plant.
Contractors
Fläkt Woods was awarded a project to supply the air-handling solutions on the newly started Hillerød plant. The contract was the largest ever air handling unit turnkey contract undertaken by the company and proved the European capabilities of the Fläkt Woods Group.
Appointed contractors carried out the installation at Hillerød and were available throughout the warranty period of the equipment to provide support. The new manufacturing facility has 29 air handling units from the EU range of Fläkt Woods, as part of a contract valued at nearly €700,000.
AHUs ranging from 4.0m³/s–33.0m³/s have been provided; the largest unit is 4m wide by 3.5m high. The large-scale manufacturing building construction project and other support infrastructure also involves the engineering services of ARUP along with Birch & Krogboe. Rockwool Denmark is providing some of the insulation and construction materials for the buildings.
HQ
NCC Construction was awarded the construction contract for new head offices and laboratories at Hillerød in early 2005. The contract was worth SEK191m.
Biogen Idec, the world’s third largest biotech company believed that it needed to have new Danish head offices near to its new large-scale manufacturing facility. The contract was for the construction of three buildings that contain laboratory, administrative and restaurant facilities.
Floor space in the buildings is 10,231m². The architect was Vilhelm Lauritzen and the advisers were Birch & Krogboe, both of Denmark and Jacobs Engineering of the United Kingdom. Fluor, a North America company, and Novo Nordisk Engineering of Denmark also represented in construction management.
The construction project was completed on schedule by the first quarter of 2006 and the buildings have now been occupied, providing an ideal base for coordinating the completion of the new large-scale manufacturing facility.
Pharmaceutical manufacturing
Once complete, the large-scale pharmaceutical manufacturing plant will be one of the largest in the world with over 90,000l of bioreactor capacity housed inside a 34,000m² facility.
The facility will consist of six 15,000l bioreactors designed to be used for mammalian cell line batch processing. These will be installed in a multi-modular fashion allowing their entire capacity to be used to produce one product, or for each one to be used for separate products if required.
In addition the facility will incorporate a 240,000m² warehouse facility, a second purification train and extensive laboratory facilities for quality control.
The downstream processing facilities to service six bioreactors of this size are currently under discussion and installation is imminent.
Packaging and quality
In May 2007 the Biogen Idec packaging facility at Hillerød was completed. The company relocated its entire packing facility and quality laboratory to the Hillerød site. Product packing and quality assurance, which was previously based in the Dutch town of Hoofddorp was also moved so that the entire production process could be based in Hillerød.
The Dutch packing facilities were moved to Denmark together with the related planning, logistics, and distribution facilities/functions/departments. It involved the transfer of more than 150 Dutch jobs and more importantly the employment of a further 300 new employees. The new packing facilities distribute Biogen Idec’s products to more than 90 countries throughout the world. The packing facilities primarily distribute medicines for use by patients who are diagnosed with multiple sclerosis.
Birgitte Thygesen, vice director of Biogen Idec Denmark, said: “From our point of view it is an advantage that we can hold on to as many Dutch employees as possible. This will ensure an efficient start-up, as they possess the necessary knowledge to get things under way.”
Products
One of the first drugs to be produced at the plant will be Tysabri (Natalizumab) (previously Antegren), a drug for the treatment of multiple sclerosis. Natalizumab is a monoclonal antibody against integrin-α4 that has proven efficacy in the treatment of two serious autoimmune disorders: multiple sclerosis (MS) and Crohn’s disease (CD).
Biogen Idec’s relapsed multiple sclerosis treatment Avonex (human interferon beta-1a) will also be produced. The psoriasis treatment Amevive (alefacept) is also due to be produced at the new plant. Biogen Idec purchased Conforma Therapeutics Corporation in May 2006 for $150m to obtain their early-stage cancer-fighting technology advances. They also licensed several promising compounds from Protein Design Labs Inc in 2005 in a deal that could eventually be worth about $660m.
The Biogen Idec drug pipeline is constantly being changed and the Hillerød plant is designed to be flexible and able to incorporate changes in production strategy very rapidly.
Biogen Idec officials have conceded that the new plant’s production costs will be about the same as using third-party manufacturers. Biogen Idec’s CEO James Mullen commented: “Building our own plant will give us greater flexibility and control of the finished product and enhance our presence outside the US.”
He added that the company’s manufacturing capacity has attracted other biotech and pharmaceutical companies to contact Biogen Idec, proposing joint ventures or technology licensing. Birgitte Thygesen, Associate Director, Manufacturing Administration for Biogen Denmark commented that the plant was mainly for the company’s own production but it was possible it could be used in joint ventures with other companies or for contract manufacturing.
Medicon valley workforce
The plant will provide 400 potential new jobs to the Hillerød municipality. However, the geographical situation of the facility site in Medicon Valley (on the border of Denmark and Sweden, connected by the 16km Oresund Bridge) has led Biogen Idec to estimate that half the new workforce will come from Denmark with the remainder from Sweden. The new plant will increase Biogen Idec’s employment worldwide by more than 20%. There will also be an influx of Dutch workers as the packaging and quality functions are being transferred to Hillerød.