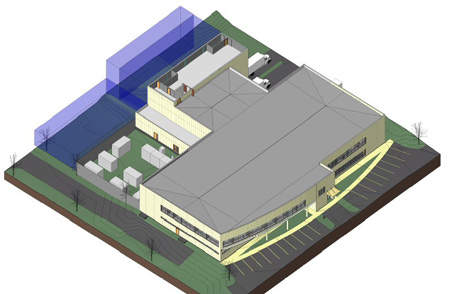
Biopol Laboratory, a subsidiary of the ALK-Abelló Group, completed the construction of a new manufacturing and research and development facility in Post Falls, Idaho, US in 2009.
The facility has been built on a 12.5-acre site in Riverbend Commerce Park, south of Interstate 90. It is the main office and the largest production site of raw materials for the company’s allergy immunotherapy products.
The facility required an initial investment of around $30m, and provided approximately 50 jobs.
Miles Guralnick, president of Biopol Laboratories, commented on the project: “We are excited to be moving to this new dynamic region in Northern Idaho. Through the planning and design phases we have built very good relations with the Idaho and regional governments and business communities, and I expect these relations to develop into a mutually beneficial cooperation.”
Facilities
Biopol Laboratory, which has been producing immunotherapy drug compounds since 1977, operated out of five facilities in and around Spokane, Washington. It also has use of a 600-acre farm in Plummer, Idaho, where it grows a variety of grasses and other plants to produce pollens, which are used as raw materials for allergy immunotherapy products.
The idea behind the new plant was to rationalise and centralise production of raw materials at one site to increase efficiency. In 2009, Biopol relocated its lab, offices, manufacturing facilities, and associated in-process and finished product warehouses to the new site.
Compound production
The initial phase of construction covered ALK-Abelló’s raw material needs for immunotherapy tablets for grass and house dust mite allergies, as well as some of the existing subcutaneous and sublingual immunotherapy products for the US and European markets.
The second phase included raw material for future products such as a tablet immunotherapy for ragweed pollen allergy, which is currently in the planning stage of a large-scale development programme. Further clinical development of the tablet has been undertaken in collaboration with ALK-Abelló’s US partner, Schering-Plough, which has been merged with Merck.
Construction
Integrated Project Services (IPS) was contracted to provide design, product development and process engineering services, and to ensure validation in accordance with latest cGMP and cGLP requirements. The firm broke ground in July 2007, following permissions granted at city, county and state level.
The new facility has increased production capacity for Biopol’s products, and allows scale-up for production of its allergen source materials.
The site also includes new pollen and mite processing buildings, as well as energy efficiency for process and utility systems, odours / dust control systems and waste treatment.
Miles Guralnick said: “IPS is the perfect partner for Biopol. They have a high level of competency and the experience required for the detailed design of this bulk pharmaceutical facility… the Post Falls facility offers the additional space and increased capacity to enable us to continue to provide an uninterrupted supply of quality allergenic biological source materials. We look forward to a good collaboration and to a very successful project.”
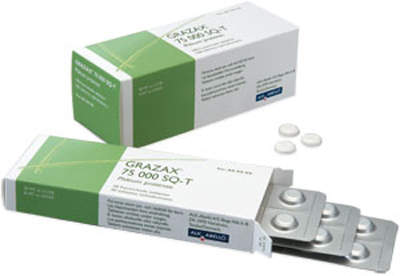
Grazax
Grazax is the first tablet-based sublingual immunotherapy treatment for grass pollen allergy to be developed for approval across Europe. It received approval in 27 European companies in 2009.
The drug has completed a special clinical trial (GT-08) for patients with a greater than two-year history of seasonal allergic rhinitis.
In June 2006, the GT-08 study made preliminary findings and reported highly significant results (P<0.0001), with reduction in rhinoconjunctivitis symptoms (43%) and use of symptomatic medications (68%) from the very first day of the first grass pollen season with Grazax treatment compared with a placebo.
In addition, 82% of patients treated with Grazax responded to the treatment, a 49% increase compared with the placebo group. The study found that the treatment dramatically reduced rhinoconjunctivitis symptoms such as sneezing and eye and nose irritation. Most participants also considered the treatment to be convenient.
Professor Dr Claus Bachert of the ENT Department, The University of Ghent commented: “We are very excited by these important results from GT-08. While symptomatic medications for treating the eye and nose symptoms of allergy are generally available to grass allergy sufferers everywhere, the accessibility to causal immunotherapy has been limited.
The widespread variations in the availability of specialist allergy services across Europe have limited access to effective treatment for grass allergy to only very few people with many waiting in line. With Grazax, we now have a treatment that can be quickly and simply administered at home by the patient.”
GRAZAX was approved in 2009, following the positive results of the first follow-up year. By the end of 2009, 15 clinical trials had been carried out on GRAZAX, ascertaining the safety and efficacy of the drug, and the following February, results from the second and last follow-up year confirmed that the drug had disease-modifying effects after a three-year treatment regimen.
ALK-Abelló also began the GRAZAX Asthma Prevention clinical study in 2009. The study will determine the efficacy of the drug in preventing the occurrence of asthma and new allergies in children and teenagers. The study, which will last for five years, is expected to complete the scientific documentation of the drug.