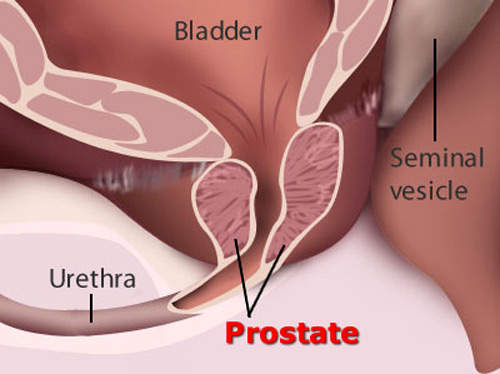
Dendreon is a US biotechnology company based in Seattle in the spotlight over its controversial cancer vaccine for the treatment of prostate cancer, which is the third most prevalent cancer worldwide, causing over 40,000 male deaths a year in the US and UK.
In 2005 Dendreon began the construction of a new manufacturing plant in New Jersey to produce its investigational active cellular immunotherapy (Provenge vaccine) for advanced prostate cancer.
The plant was topped out in August 2006 with the fit-out completed in June 2007.
In May 2007 the US Food and Drug Administration (FDA) proved less enthusiastic about Dendreon’s new treatment than expected, which led to a scaling down of the manufacturing plant from 48 to 12 clean rooms (with associated laboratory support) and a drop in company share prices.
The FDA issued Dendreon an approvable letter but it was asked to present more data for the efficacy of Provenge. A 500-patient clinical trial was conducted. Results of the Phase III trial of Provenge were announced in March 2009, and the FDA approved the drug in April 2010.
This Phase III clinical trial was designed to show that Provenge extends life expectancy when given to men whose prostate cancer has metastasised to other parts of their bodies. These cases would not have a good prognosis with existing treatments. The trial proved the safety and efficacy of the vaccine.
Provenge vaccine for prostate cancer treatment
Dendreon has been working on the development of novel therapeutics that harness the immune system to fight cancer. The company has strong experience in antigen identification, antigen engineering and antigen-presenting cell processing and this has led to compounds capable of causing a cell-mediated immune response.
Provenge (sipuleucel-T) is the company’s lead active cellular immunotherapy that was tested in the recently completed Phase III development for treatment of advanced hormone-refractory prostate cancer. Provenge targets the prostate cancer antigen, prostatic acid phosphatase (PAP), which is found in approximately 95% of prostate cancers. Dendreon also has Trp-p8 in preclinical development, a cold receptor and transmembrane ion channel, which is over-expressed in breast, prostate, lung and colon cancers.
Dendreon plant construction
The new plant was constructed by the Henderson Corporation with architectural services supplied by Perkins & Will. Although the 158,242ft² plant construction was completed in August 2006 the fit-out was scaled down in June 2007.
To increase capacity and quickly manufacture sipuleucel-T, the facility is now being expanded. During the expansion, the facility will employ a flex design concept, which will allow consistency, flexibility and easy replication of the manufacturing space including workstations, quality control and support areas.
The clean rooms will incorporate a modular design. The design allows the rooms to be prefabricated with materials that are easy to sanitise before it is placed into the large warehouse-like space that makes up the facility area. It will enable easy replication, quick installation and simple validation.
In March 2011, the FDA approved the addition of 36 workstations, which will quadruple the plant’s Provenge production capacity. This will bring the total number of workstations to 48.
Provenge technology
Provenge is delivered using proprietary antigen delivery cassette technology, which uses a recombinant form of an antigen found in approximately 95% of prostate cancers, prostatic acid phosphatase (PAP), to stimulate the patient’s immune system to attack the cancer cells.
In March 2006 Diosynth was awarded a long-term contract by Dendreon to produce the recombinant protein antigen component of the new Provenge therapy.
Diosynth had already been producing the recombinant protein antigen component of the product on a small scale to supply clinical trials from its North Carolina plant and scaled up manufacturing capacity to meet expected commercial demand.
Licensing
In 2006 Provenge was given a Fast Track designation and Dendreon submitted a Biologics License Application (BLA) to the FDA as a rolling submission for approval to market. They intended to apply for Priority Review upon completion of the BLA submission.
The company received notice of approval from an FDA panel in March 2007. However, following criticism from two experts that the clinical studies were too small, in May 2007 the FDA asked for more evidence.
With the Phase III results being positive, Provenge is expected to become a potential blockbuster treatment for prostate cancer.