In June 2007, Genzyme Corporation announced the construction of a new biomanufacturing plant in Lyon, France. The company has more than 320 employees in France at various sites, including its existing production plant located in Marcy l’Etoile (Lyon) and its regional headquarters in the Lyon-Gerland area.
The Marcy l’Etoile plant handles the exclusive production of two drugs in the field of transplantation, but has become too small for the production volume that is required.
The new biomanufacturing plant will replace the Marcy l’Etoile plant and will have more than twice the production capacity.
Frédéric Turner, senior vice-president and general manager for France and North Africa said: “This investment sends a clear message of support for French research and biotechnology and it illustrates Genzyme’s culture of establishing strong partnerships in the regions where we operate.”
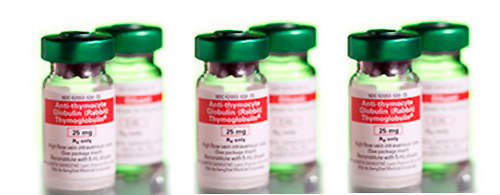
Production of Thymoglobulin
The new plant has been built for the production of Thymoglobulin, an anti-thymocyte globulin agent used in transplantation procedures. Thymoglobulin is a polyclonal antibody extracted from rabbit serum. It is used to treat or prevent acute rejection in various transplants, treat severe or steroid-resistant acute rejection in kidney transplants and treat aplastic anaemia.
Dr A Osama Gaber, MD, a professor of surgery and director of transplantation at the University of Tennessee, commented on Thymoglobulin after its approval in 1999, calling its availability in the US a major advance for transplant recipients.
“Acute rejection is one of the most significant risk factors in determining the success of a new organ and a kidney recipient’s survival,” he said. “Clinical trials show that Thymoglobulin can be a very effective and safe therapy for these patients.”
The new facility is needed to meet the anticipated long-term demand for Thymoglobulin, both for current uses and also in potential new indications. The growth in the use of Thymoglobulin is being driven by its launch in new geographic markets and by positive publications and clinical studies. The market for the drug in 2004 was in excess of $90m and Genzyme sales in 2006 increased by 17% – up to $149m – compared with 2005.
The product is approved and marketed in more than 55 countries for various indications, including the treatment and the prevention of acute rejection of solid organ transplants. Thymoglobulin has potential beyond the transplantation field, with recent studies exploring its use in the treatment of blood disorders and autoimmune diseases such as diabetes.
New plant
The new 22,000m² facility is located in the Lyon-Gerland Biopôle area on a 3.7ha site, which will allow Genzyme to expand the plant if required. The total cost of the project is in the region of €105m.
The company expects that approximately 50 new jobs will be created when the new facility is operating at full capacity. It is intended that the smaller Marcy l’Etoile plant will close and its 165 employees will be transferred to the new plant.
Construction began in April 2008, and the first phase of transfer activities took place in April 2010. More than 100 employees have been moved to the new site so far, and production activity will be transferred in the coming months.
Subject to regulatory approvals, the facility is expected to begin production in 2011.
Mark Bamforth, senior vice-president for corporate operations, said: “As with our other new facilities, we intend the Lyon plant to serve as a highly visible expression of Genzyme’s purpose, which is to innovate and set new standards in both the products that we develop for patients and in the way that we operate as a company.”
Environmental issues
Genzyme has a new resolve and commitment to innovation and sustainable development. This new facility has an attractive and contemporary architectural design and uses state-of-the-art green technology to provide a comfortable atmosphere while reducing the impact the plant has on the environment.
Genzmye developed the site using a High Environmental Quality (HEQ) approach by obtaining HEQ certification through meeting or exceeding standards set by the Center for Scientific and Technical Building. The plant is one of the first manufacturing sites in France to gain HEQ certification, which is similar to the certification provided by the US Green Building Council through its LEED (Leadership in Energy and Environmental Design) green-building rating system. The French Health Products Safety has also authorised the opening of the site.
Design and construction
The preliminary design of the Lyon facility was completed by the French architects Patriarche & Co in collaboration with Genzyme. SNC Lavalin has provided engineering and design services for the project.