Pfizer Manufacturing Deutschland GmbH is a large secondary manufacturing centre for Pfizer in Germany and has a reputation for efficiency, high containment and economy. The facility in Illertissen needed to increase its capacity in early 2006 to accommodate the growing market demand for the newly developed smoking cessation drug Chantix.
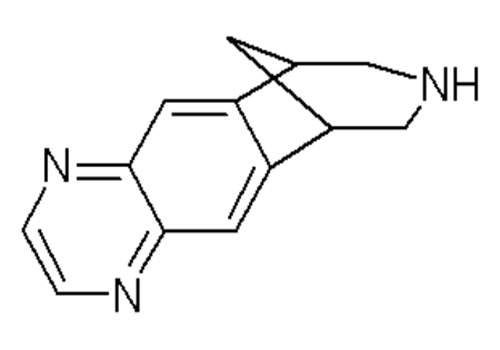
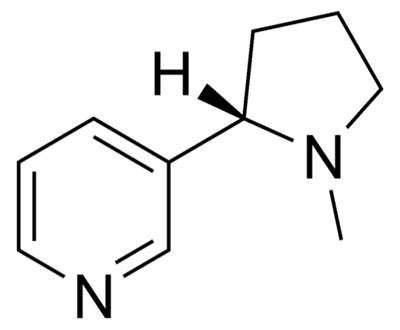
The drug was first released to the commercial market in 2006 and Pfizer at Illertissen was the secondary production plant chosen to produce the drug from the highly potent API varenicline, which is produced in Ireland at the Little Island plant.
The problem was there was a lack of capacity at the Illertissen plant for producing solid dosage forms under high containment, such as was required for Chantix.
Because of this the NEWCON facility was conceived, planned and constructed in a short timescale between 2006 and 2008. In addition, Pfizer wanted to make the process more efficient by cutting the requirement for staff to use high containment suits during their day-to-day activities.
In October 2008 the NEWCON facility was named as the International Society of Pharmaceutical Engineers Facility of the Year 2008 overall winner.
Pilot scale
Prior to the smoking cessation drug being brought to market, the Illertissen plant had already developed a pilot-scale high containment facility in 2003 inside the existing building, which was called ICON (Illertissen Containment).
This facility demonstrated for the first time many of the new techniques, equipment and principles, which would be needed in the larger NEWCON facility.
ICON introduced dust-free and fully automated production. The features of this system included: a single room concept with a hermetically sealed containment module; state-of-the-art isolator technologies; transport of hazardous substances by vacuum systems and split-valve containment technologies; and the use of laser-guided driverless vehicles, between weighing, granulation machines, tablet presses and coaters. NEWCON was designed on these principles.
Construction
The new containment facility for oral solid dosage started construction in 2006 and was designed by PhC Pharmaconsult of Heidelberg, who acted as architect and consultant for the project.
The project and construction management were carried out by Pfizer itself using the experience of the team at Illertissen. The facility cost €35m ($55m) to construct and outfit and was completed six months ahead of schedule in October 2007.
The new facility is 83,958ft² (7,800m²) in floor space and contains state-of-the-art containment technology. The NEWCON facility is a single-floor, single-containment module that is able to house up to three production trains, with the first train currently operational for the production of Chantix (Varenicline).
The concept of NEWCON uses a through-the-wall design where all production areas are screened off with a transparent containment screen inside a dedicated processing module; the driving machinery is located outside these areas for easy access for maintenance and repair.
The maximum workplace exposure to the API varenicline (dust/particle exposure) is set at 1–10µg per m³ but the NEWCON facility is considerably within this, only allowing a maximum of 0.1µg per m³. This level of containment has made it possible to undertake near dust-free production and a fully automated production of the new film-coated tablets. It is one of the first containment facilities across the world to adopt this level of automatic control.
All of the critical process stages can be monitored and controlled from a separate control area so that employees are not exposed to any dust that may be generated during the tablet production run. The production stages have been designed so that there are much lower frictional losses and there are far fewer employees.
The facility makes full use of process analytical technology (PAT) to carry out quality checks on the products. For example, near infrared (NIR) spectroscopy is used to confirm the homogeneity of batches and the presence of the active ingredient. Other PAT technology is used to check weight, hardness and diameter of the tablets.
The facility also uses the concept of lean manufacturing with three batches being processed simultaneously in different parts of the building. The three-shift system and semi-continuous production allows over a billion tablets to be produced every year.