PharmStar Pharmaceuticals Inc is a relatively new drug manufacturer, which is headquartered in Rocky Mount, North Carolina. The company has been engaged in research and development since 1993 into the production of a new commercial form of soluble aspirin. In August 2008 the company opened the first phase of a new manufacturing facility at its 17,000ft² headquarters building containing labs and pilot plant, which are FDA and CGMP compliant, in the town of Rocky Mount.
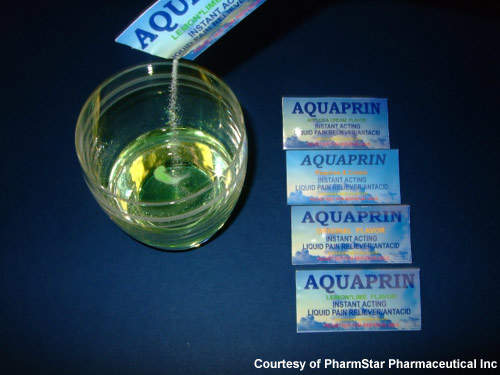
The new facility, which is part of a 196,000ft² building on Wesleyan Boulevard, will be engaged in producing commercial quantities of Aquaprin. This is a fruit-flavoured aspirin formulation which contains potassium bicarbonate and rapidly dissolves in water to give the potassium salt of acetylsalicylic acid. Unlike aspirin itself the formulation provides an easy-to-ingest drink which causes no gastric bleeding and acts as an antacid instead.
PharmStar Pharmaceuticals Inc has been actively working on the validation, manufacture and continuous product improvement of Aquaprin since 1993 and has several patents relating to the manufacture of the product. The patent protection means that, subject to FDA regulations relating to over-the-counter medicines and aspirin, the company expects to enjoy a 20-year exclusive franchise.
PharmStar intends to carry out some clinical trials on Aquaprin to satisfy the US FDA and FTC about its marketing claims of ‘fastest-acting, safest and most potent analgesic on the market’.
New facility
Howard Phykitt is an ex-nuclear engineer who has been the driving force behind the company for the past 20 years. He has seen successful versions of soluble aspirin in Europe based on calcium and sodium salts, but nothing based on potassium salts, which he believes is a unique formulation. The potassium salt of aspirin is likely to be the first of many soluble pain-relieving products from PharmStar.
Howard Phykitt commented: “The first phase of money has been raised, enabling us to produce the product, but only in the area of a few million dollars a year in sales… I’ve got this product perfected now. It is fully developed… There is no potassium-based aspirin in the world. There are sodium-based ones and calcium-based ones in Europe, which are very successful products.”
An earlier version of Aquaprin (protected with three patents) was not a success after being marketed through a national broker network in the US. The new version, perfected in late 2007, will be sold in small foil packets containing a single granular dose for mixing with water. The facility will employ 25 personnel in the first year of production and will then expand into a new 100,000ft² plant on the same site in phase two when there will be an additional 150 personnel employed. Personnel are being trained at Nash Community College and production personnel are expected to be hired within 12 months.
The Aquaprin product will be in stores for sale in around 15 months (by November 2009). Aquaprin will be manufactured in accordance with the current FDA Analgesics Monograph in the ‘permitted combinations’ section of the Federal Register and in the USP under the ‘Effervescent Aspirin’ monograph.
Development
Aquaprin will come in several flavours including peach, apple and cream, raspberry, strawberry and lemon lime. The marketing will be targeted at providing instant pain relief and aspirin prophylaxis against heart attack and stroke. Other versions in development are a pain-relieving sports drink and the Instaprin device, which will be a single-use system carried by the user containing 1.5 ounces of water and a dose of potassium aspirin for mixing and use on the move.
Research has shown that use of prophylactic levels of aspirin can have great benefits for sufferers of a stroke or heart attack and this is one of the main applications for the Instaprin device.
Clinical
The formulation instantly dissolves the aspirin on contact with water, making it a fast-acting, non-acidic, liquid derivative of aspirin. In this form, it virtually eliminates irritation to the GI-tract, and is a more effective and efficient method of delivering salicylates into the blood stream. This form of aspirin can achieve peak blood plasma levels of the drug in five to seven minutes.
In addition, it contains therapeutically active levels of potassium, which act as an antacid in neutralising stomach acid and also lower blood pressure and help to dilate blood vessels allowing for better circulation.