Rubius Therapeutics is constructing a pharmaceutical manufacturing facility in Smithfield, Rhode Island, US.
The plant will use Rubius Therapeutics’ erythrocyte design platform RED to scale-up the manufacturing of Red Cell Therapeutic™ (RCT) product candidates.
The company intends to invest up to $95m on the facility by 2020.
An official ground-breaking ceremony for the project was held in September 2018 and the first phase of construction is expected to be completed by 2020. The project will create approximately 160 new jobs in Smithfield.
Rubius’ new pharmaceutical manufacturing site location
In July 2018, Rubius purchased an existing 135,000ft² building from Alexion Pharmaceuticals for the project.
Located at 100 Technology Way and 30 Hanton City Road in Smithfield, the building will be renovated to be used as the company’s new pharmaceutical manufacturing facility.
Rhode Island has emerged as a favourable location for various companies due to the incentives offered by the Qualified Jobs Tax Credit Program.
Rubius’ new pharmaceutical manufacturing facility details
The customised facility will be equipped with multiple current good manufacturing practices (CGMP) compliant manufacturing suites for clinical supply.
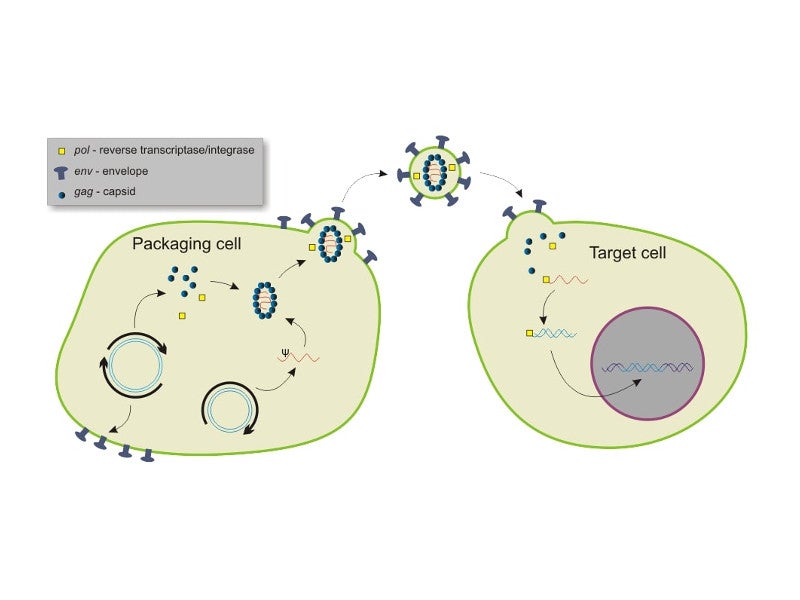
The Red platform will be used to generate genetically engineered RCT product candidates at the facility.
RCT can be used for the development of potent and allogeneic cellular medicines that can potentially treat a number of patients from a single universal donor. It can develop medicines for a wide range of diseases such as phenylketonuria, refractory gout, homocystinuria, hyperoxaluria, solid tumours, pemphigus vulgaris, and type-1 diabetes.
The facility will also be utilised for the commercial supply of the RCT product candidates after approval. It will enable the company to ensure the availability of medicines for patients as soon as they are required. The lentiviral vectors required to code biotherapeutic proteins in the RCT will also be developed in the facility.
Financing
Gina Raimondo, the Governor of Rhode Island, and the Rhode Island Commerce Corporation worked closely with the company to provide incentives for the project.
The Board of the Rhode Island Commerce Corporation approved tax credits valued at approximately $2.75m through the Rebuild Rhode Island Tax Credit Program and the Qualified Jobs Incentive Act.
Qualified Jobs tax credits worth approximately $370,000 a year will be provided over ten years. A total of $9.345m-worth credits over the ten-year period are expected to be provided.
Rubius is also eligible to obtain sales tax rebate on the construction materials utilised in the project.
Contractors involved
A/Z Corporation has been appointed to design and develop the new facility. It will collaborate with other design partners to draft the required renovations to develop the facility.
Marketing Commentary on Rubius Therapeutics
Rubius Therapeutics was founded in 2013 by Flagship Labs, a life science innovation enterprise founded by venture capital firm Flagship Pioneering.
The company was established on the discoveries of the scientists of Whitehead Institute for Biomedical Research at MIT and research findings of Flagship Pioneering’s VentureLabs.
Rubius focuses on the advancement of the RCT product candidates by utilising three types of therapeutic modes, which include cellular shielding, cell-cell interaction, and tolerance induction.