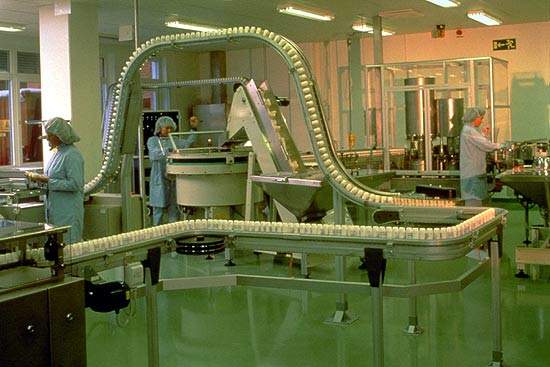
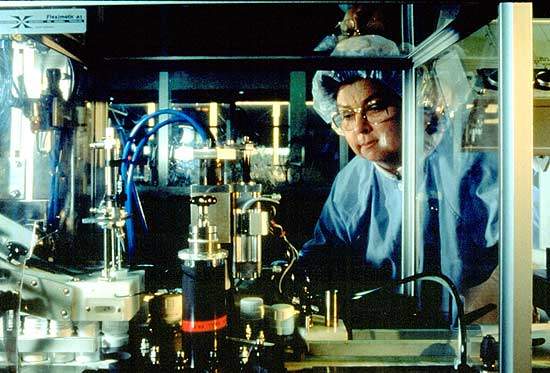
AstraZeneca’s pharmaceutical plant in Snackviken, Södartälje, Sweden was formed during the merger of Astra and Zeneca in 1999.
The Södartälje facility served as Astra’s headquarters until the merger, when Astra moved its head office to London.
Designed to help meet growing demand for the company’s Turbuler range of asthmatic inhalers, the pharmaceuticals complex had considerable economic importance for the surrounding area and was part of an investment programme that provided around 1,000 jobs over a period of four years to 2005.
Södartälje is now the research and development (R&D) headquarters of AstraZeneca and employs more than 8,000 people. With around 1,600 employees, the site undertakes research focussed on neuroscience. The company is planning to cut 1,400 jobs in Europe by 2013.
Asthma treatment production plant in Sweden
The Snackviken plant manufactures asthma treatments Oxis and Pulmicort. Oxis is a long-acting beta2-agonist with a fast onset of action. It is designed as a once or twice-daily maintenance therapy for asthma and an anti-inflammatory treatment with inhaled glucocorticosteroids.
Pulmicort is an inhaled anti-inflammatory glucocorticosteroid, which is primarily used in once or twice-daily maintenance asthma treatments.
The Turbular product is a chlorofluorocarbon (CFC) free, multidose dry-powder inhaler.
Snackviken plant expansion
An expansion of the Snackviken plant began construction in 2000 and went into commercial production in 2003.
It involved an investment of around $94m, which was part of a wider $600m investment programme. Head Engineering procured production lines and provided project management services for the expansion.
AstraZeneca also planned to build a new tablet plant in Gartuna, Södartälje, which was estimated to be completed in 2001 at a cost of SEK1.4bn ($162m). The groundwork for the project was completed, but further work was suspended in February 2000.
The Gartuna facility was stopped following a decision by the US Food and Drug Administration (FDA) to allow AstraZeneca to bypass a stage in the production of Plendil ingredient metoprolol, which created spare capacity at the group’s plant in Snackviken. This was more economical for the company to use and cheaper than building an entirely new plant.
Projects at AstraZeneca’s pharmaceuticals plant at Södartälje, Sweden
An additional 13,500m² plant was built at the Snackviken site in 2003 for the production of Nexium IV. This included a 2,400m² microbiological analysis laboratory. SWECO FFNS designed the plant, while Head Engineering validated the facility.
Head Engineering also installed a -35°C emergency super cooling plant at the site. This SEK45m ($6.5m) project was completed between 2004 and 2006.
In September 2007, a new packaging line was started at the plant. It is installed with FlexPack systems and robotics automation supplied by ABB. The line reduces packaging and the time-to-market of an anti-inflammatory glucocorticosteroid called Rhinocort, used for the treatment of respiratory diseases.
In 2010, the end-of-line ABB Robotics pack-handling systems were installed at the facility to pack Nexium. It has four dedicated Nexium lines and blister-packing machinery supplied by Uhlmann and Bosch Packaging.