Colorectal cancer (CRC) is the third most common type of cancer in the US, and its treatment is often associated with severe side effects.
According to the American Cancer Society there were 135,000 new cases of CRC nationwide in 2016 and an estimated 49,000 deaths attributable to the disease.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
A promising area of drug development is cellular immunotherapy, which forces the immune system to find and destroy a specific cancer-causing target.
Blockbuster treatment
The most commonly prescribed therapeutic, Avastin, is a targeted therapy that entered the market in 2004.
Roche’s product has been highly successful, making $7 billion in 2016.
However, the drug’s safety profile is poor, and patients often experience serious side effects, such as hypertension and bleeding.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPromising alternative
There are two vaccines in the late stages of development for CRC, of which MelCancerVac has the most promising trial data.
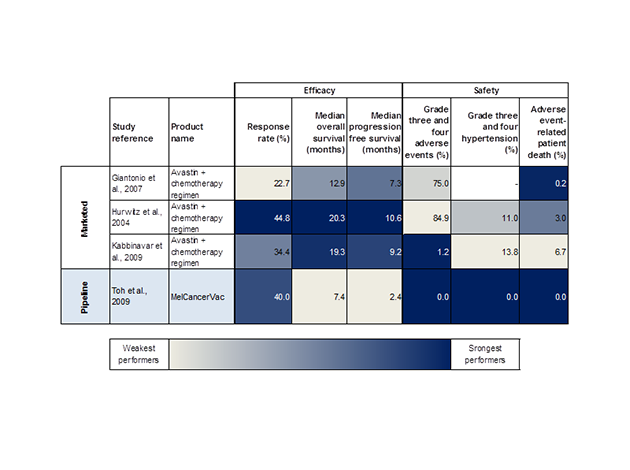
Clinical Trial Data, Comparison of Efficacy and Safety Data Endpoints for Avastin (bevacizumab) and MelCancerVac

Source: GBI Research Pipeline Products Database, Colorectal cancer
Zero side effects
MelCancerVac has a far superior safety profile to Avastin; in its pivotal Phase II study it showed comparable effectiveness, but there are so far no reports of any severe adverse events or patient-related deaths.
In contrast, as many as 84.9% of those receiving Avastin experienced severe side effects, and 6.7% died as a result of these.
Limited effectiveness as monotherapy
Not all the results for MelCancerVac have been equally promising; patients did not survive as long on average.
However, in the studies Avastin was co-administered with a chemotherapy regimen, inevitably improving the drug’s performance.
MelCancerVac’s effectiveness may well improve when it is used alongside chemotherapy in later-stage trials.
Testing on a larger group of patients would also help validate the data, as only 20 metastatic CRC patients were observed, compared with 800 in the Avastin studies.
Potential to transform cancer treatment
MelCancerVac and cancer vaccines like it are widely considered to make up the most innovative and high-growth area within the biotech space.
The next step will be to develop more effective vaccine programs that are effective on a large scale – for MelCancerVac this could involve experimenting with different combinations of products or regimens for it to be administered with.
Methods are continually improving as cancer knowledge advances and, if successful, this form of therapy could drastically alter the way CRC is treated.




