Adoptive cell therapy (ACT) is a form of cancer treatment that involves the transfer of immune cells into a patient to help fight the disease, by eliminating cancer cells. These cells can be taken originally from the patient or another individual. The concept behind this form of treatment is that the introduction of these cells will enhance the immune response to disease, often through bioengineering the immune cells to have improved functionality and characteristics, such as longer half-lives.
Chimeric antigen receptor T (CAR-T) cells are a form of ACT in which cells are engineered to express a synthetic receptor on their surface that involves a recognition domain derived from antibodies and signalling domains derived from T cell surface receptors (TCRs). They were first licensed for use in 2017 for two forms of blood cancer, acute lymphoblastic leukemia (ALL), and B-cell lymphoma (BCL).
Overall, the global CAR-T cell market for all indications was worth $1.7 billion in 2021 and is forecast to reach $2.4 billion by the end of 2022. By 2028, the market is expected to reach $25 billion, with a compound annual growth rate (CAGR) of 46.6%. This makes CAR-T cells one of the fastest growing and potentially profitable therapeutic areas in oncology.
One key hematological indication in which CAR-T cells have been used as an effective therapeutic is diffuse large B-cell lymphoma (DLBCL), which occurs mostly in women. According to GlobalData’s Epidemiology database, there were 5,446 incident cases of DLBCL in the UK in 2021. The standard first-line treatment for DLBCL is a chemotherapy regimen, alongside the monoclonal antibody rituximab. However, the relapsed/refractory (R/R) rate for patients can be up to 50%. CAR-T cells are an exciting development in this area because, prior to their development, the prognosis for these R/R DLBCL patients was very poor, with no other effective treatment options. CAR-T cells produce high efficacy in this population and are therefore key for improving disease outcomes in this indication.
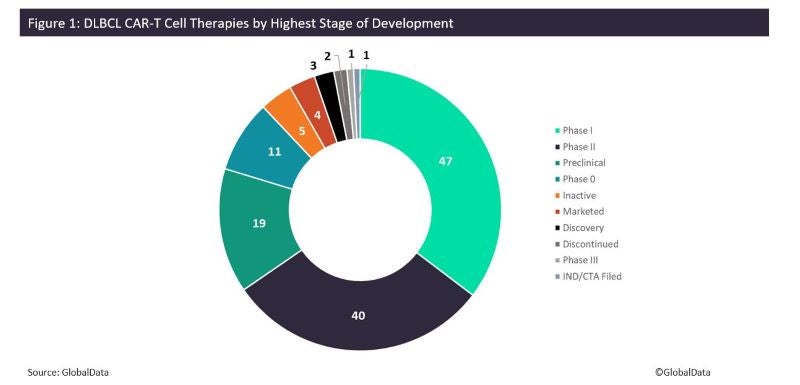
According to GlobalData’s Drugs database, there are currently 133 different CAR-T therapies in total for this indication worldwide, including four marketed drugs (Figure 1). The two major players in this field are Gilead and Novartis, which have developed two pioneering CAR-T cell therapies, namely Gilead’s Yescarta (axicabtagene ciloleucel), and Novartis’s Kymriah (tisagenlecleucel). These therapies also have other marketed and pipeline indications, including other blood cancers and myeloma.
Other, more recently marketed CAR-T cell therapies for DLBCL are Juno Therapeutics’ Breyanzi (lisocabtagene maraleucel), and JW Cayman Therapeutics’ Carteyva (relmacabtagene autoleucel). Each of these approved CAR-T cell therapies consist of cells derived from the patient’s blood and target cancer cells expressing B lymphocyte antigen CD19.
The DLBCL CAR-T cell therapy pipeline consists of 129 distinct drugs spanning all stages of development. Many of these drugs are in early development, with approximately 80% in Phases 0–II. Phase I molecules constitute most of the early-stage pipeline, with 47 drugs, followed by Phase II at 40 candidates. There are also 19 drugs in preclinical development (Table 1). The single drug in Phase III development is SACD19-101 by Sian Wuhan Medical Technology, which is also in development for other indications such as chronic lymphocytic leukemia. Notable Phase II drugs include Allogene Therapuetics’s ALLO-501A, Autolus Therapeutics’s obecabtagene autoleucel, CASI Pharmaceuticals’s CNCT-19, Precision Biosciences’s PBCAR-0191, and Mustang Bio’s MB-106.
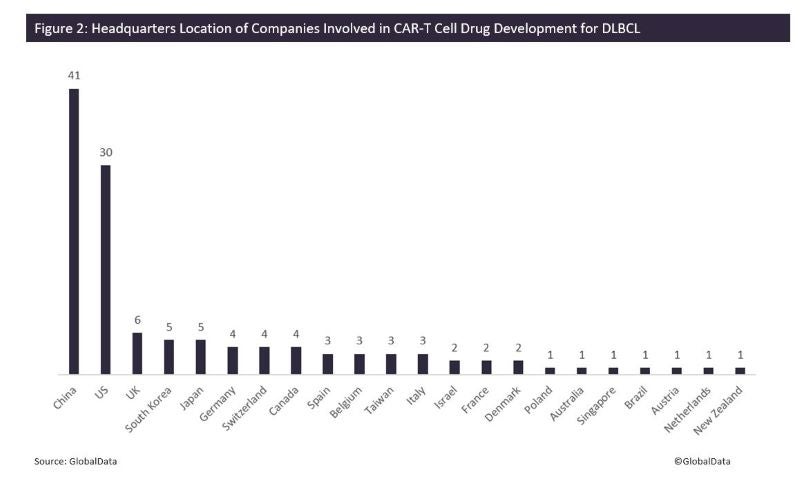
There are a total of 124 companies involved in CAR-T cell drug development for this indication. Many of the companies developing these drugs have their headquarters located in China (41 companies), followed by the US at 30 (Figure 2). This is in line with China’s increased domestic development of immune-oncology drugs in order to compete with geographic expansion of global developers to China over the next few years.
The current landscape for CAR-T cell therapies is promising, with multiple drug approvals already, a rich pipeline, and many clinical trials ongoing. However, CAR-T cell therapies have limitations such as relapse or severe autoimmune side effects. Future treatment options will hopefully offer alternatives that alleviate these concerns. Another issue is the high cost of these treatment options, incurred partly due to their personalised nature. One potential bioengineering solution is the development of universal, allogeneic CAR-T cell therapies that can be used as off-the-shelf treatments. This has shown exciting promise in limited clinical settings thus far.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.