
Drugs have very specific temperature and storage requirements that must be considered at every point in the supply chain. Temperatures can vary considerably while in transit and shipments are exposed to a variety of weather conditions during air freight, while on trucks, and in shipping containers.
Additionally, soaring temperatures, wildfires, and floods are all becoming more common because of changes in weather patterns due to climate change[i]. For temperature-controlled drugs, extreme conditions and delays in transport can have a potentially adverse impact.
Maintaining and monitoring such highly specific requirements for temperature-controlled shipments place unique demands on packaging solutions.
Secondary packaging and climate change
According to new research by GlobalData[ii], pharmaceutical packaging is experiencing significant advancements due to the need for solutions with specific requirements including on-time controlled shipments with advanced preparation and consideration.
As companies strive to reduce their carbon footprints, a growing trend in the market is the use of recycled or reclaimed plastics in packaging. Eco-friendly plastics from bio-based sources are also gaining popularity.
Managing temperature-controlled shipments in unpredictable climate zones means that secondary packaging is critical in providing temperature control for some medicines to maintain their stability and efficacy.
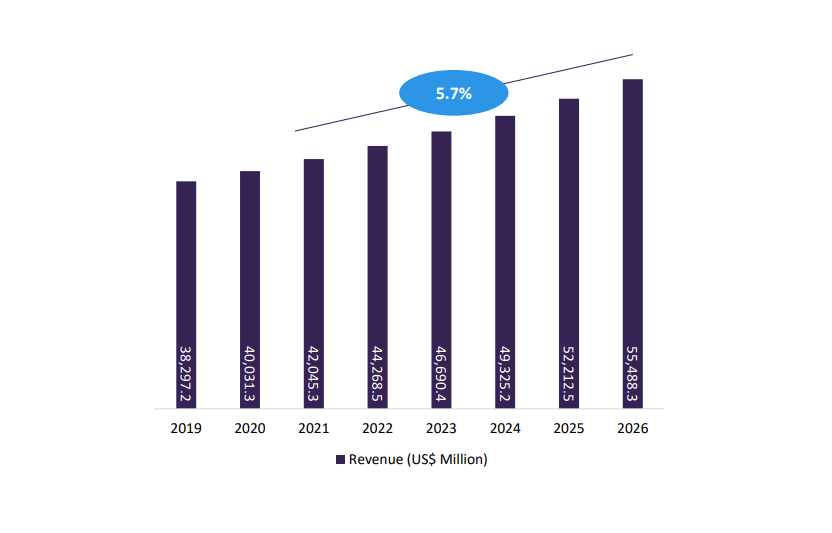
GlobalData forecasts show revenue estimates of pharmaceutical temperature-controlled secondary packaging to experience a CAGR of 5.7% from 2021 to 2026, reaching USD 5,536.3 million.
Fig.1: Pharmaceutical Packaging Market Estimates & Forecasts, Secondary Packaging: by Void-fill and temp controlled packaging, 2019-2026 (US$ Million). Credit: GlobalData
Challenges of a warming climate for medicines
Dr Simon Magness is the Group Director of Operations at BAP Pharma. He is responsible for the global oversight of the company’s operations which includes their sites in the UK, Germany, and the US.
According to Magness, providing secondary packaging solutions for new drugs with unique temperature and storage demands is becoming more complex and necessitates expert requirements.
“Each new drug can have its own unique temperature and storage requirements,” he explains. “Some of the drugs have specific temperature ranges that need to be maintained to preserve their efficacy. So, the packaging solutions must be designed to consistently maintain the precise temperatures that you need.”
Regulatory requirements also cannot be overlooked. “Ultimately, it’s about patient safety and ensuring that the drug is maintained under temperature-controlled conditions during its entire journey to the clinical sites globally,” says Magness. “We ensure that every packaging solution we use is tested and qualified and that it will meet our high standard of quality, all while ensuring regulatory compliance.”
Because of climate variability, fluctuations in temperature can be severe. In recent years we have experienced heat waves, storms, and even polar vortexes. Yet it is not just about maintaining the temperature of the shipment as extreme weather can also cause disruption to the supply chain in general, leading to delays and backlogs.
“Now, not only are you dealing with temperature changes, but you’re also dealing with potential delays,” points out Magness.
Meeting highly specific demands for clinical packaging
The combination of well-designed secondary packaging solutions with the correct processes can help mitigate many of these challenges, says Magness.
“When one of our clients comes to us with a shipment request, we plan that out in great detail,” he says.
“The first thing we look at is the temperature-sensitive product. What temperature does it need to be shipped at? Then we look at temperature mapping. Understanding the temperature profiles of the regions that our shipments will pass through. Identifying any hot spots, cold spots, and tailoring our packaging accordingly.
“We also look at what type of shippers we will need to use. Do we need insulated shippers or any extra materials, like thermal blankets, to maintain the desired temperature range? We also look at the qualification period of those shippers. Is it two days, is it five days? We assess all of that.”

Magness explains that to minimise risks during transport, clinical shipping requirements are governed by processes, SOPs, regulations, and agreements with clients. Shippers, tracking devices, and couriers are all qualified prior to use.
“We strongly evaluate what type of courier we use and only work with couriers that have experience working with specific temperature-controlled shipments,” adds Magness.
This also includes the correct labelling of the shippers with the temperature requirements and handling instructions (e.g., IATA) clearly visible for both transporters and recipients.
Of course, unexpected events can occur during shipments of clinical trial supplies, but this is when well-planned contingency measures are activated.
“For example, when there is an unexpected delay in customs, we assess whether the courier or airport has facilities where we can move the shipment for safe storage until it is cleared by customs,” explains Magness. “To mitigate such delays, permits and paperwork are completed well in advance, and goods are only moved if we have the green light from our couriers.”
Advantages of in-house clinical supply services
BAP Pharma was built on comparator sourcing services, and with increasing client demands, has built its Packaging, Labelling, and Distribution Division (PLD) to offer its clients strategic assistance while maintaining maximum flexibility. Magness says that it was the natural next step to implement packaging, labelling, and distribution to service the full supply strategy of clients.
“With a streamlined supply chain, we can integrate our packaging and distribution services,” he says. “With BAP supporting a client’s clinical trial we can help reduce potential delays and help optimise their supply strategy.”
It is important to have product ready on time, notes Magness: “It is not enough to only have your Investigational Medicinal Product packed and labelled. You also need to ensure it is distributed globally, safely and on time.”
BAP Pharma’s ability to remain both flexible and agile benefits its clients in an ever-changing landscape.
“We conduct very thorough planning,” Magness concludes “to ensure your product is getting to the right place, at the right time, and at the right temperature.”
To learn how BAP Pharma can help with your secondary packaging requirements, download the specially commissioned white paper below.
[i] GlobalData: Climate Change – Key Metrics Data: https://explorer.globaldata.com/Analysis/details/climate-change—key-metrics-data-169937
[ii] GlobalData: Pharmaceutical Packaging Report, Market Analysis and Forecasts To 2026, Published: March 2023


