by Dr Miha Možina
Buying equipment does not also give you permission to use it in a pharmaceutical environment; qualification and validation are still in your way. Their primary goal is not to prevent you from using your equipment or to increase its costs, but to help you ensure consistent quality of final products.
At Sensum, we interact with many pharmaceutical quality assurance teams on the topic of qualification and validation, as we develop and provide solutions for automatic visual inspection of end products, which need closer supervision by quality assurance than quality control systems. For more than 15 years, we have experienced different qualification scenarios, which allowed us to identify several good practices. In the following paragraphs, you can find practical insight into the process of qualification with some useful tips that might help you with any qualification project.
First things first; What is validation and what is qualification?
Validation is a larger concept than qualification and is related to processes such as the manufacturing process. It can be simply explained as a systematic approach that checks and helps processes to have expected and consistent results. Validation does not involve only equipment, but also various supplementary systems, software and people, which are part of the process.
Validation breaks down to several activities and one of those is the qualification, which is related to introducing systems to the process. The job of qualification is to make sure that a particular system is meeting regulatory requirements, industry standards and expected performance.
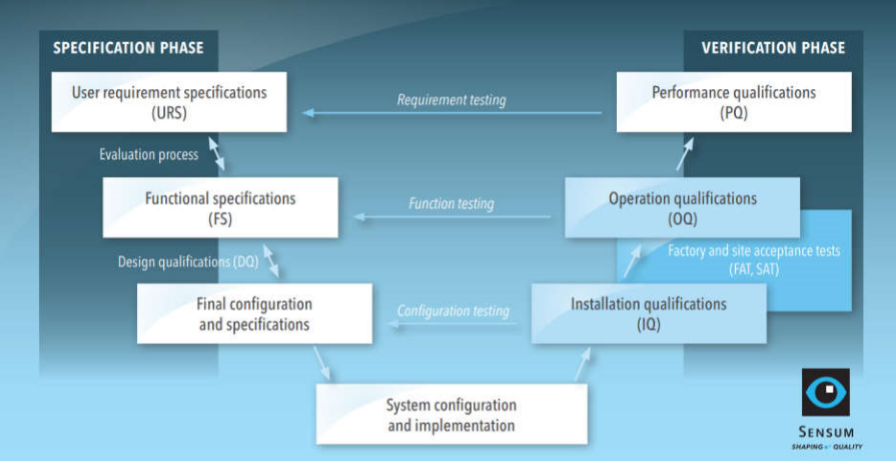
The (in)famous V-model
The extent of qualification depends on the complexity of the equipment. For example, the qualification of an intermediate bulk container should require less effort in comparison to a visual inspection system. We shall take a look at the qualification of a configured computerised system, which covers all typical qualification steps. The qualification procedure for the example is presented in the V-model below with two phases, specification and verification.

Specification phase: from URS to DQ
User Requirements Specifications (URS) are prepared by the final user who lists their expectations and requirements for their process. URS is a basic document that streamlines the entire qualification process.
SENSUM TIP: At Sensum, as a supplier, we come across many URS. Most of the URS documents have many requirements with 20+ pages, but actual requirements relevant for the specific project are written in barely one or two short points. This happens because the URS are prepared from a template or from another project’s URS without critical modifications and corrections. URS has an impact on the whole qualification procedure and cutting corners here is not helpful. For example, we recommend to remove general requirements that are not applicable; such requirements will invoke unnecessary discussion or even extend qualifications. Furthermore, do not forget to add important data. Consult your production experts so the requirements will cover the actual process needs, such as throughputs, quality of operation, efficiency, waste generation, machine settings, part assembly, downtimes, cleaning, calibration, maintenance, skills required to use the machine etc.
After the URS are agreed and approved, they are typically shared with several potential suppliers. Each supplier replies to URS with a quote, as well as Functional Specification (FS) documents, which are difficult to read and often impossible to link with each URS point.
SENSUM TIP: For faster evaluation of suppliers’ offers, make room in URS document for their comments and name the new column Functional specification, because, in fact, their comments are functional confirmations and descriptions of their machine. In this way, you can completely avoid reading through the supplier’s design documents.
Once the suppliers provide their feedback, it is time for Design Qualification (DQ). As mentioned in the introduction, the scope of qualifications depends on the system’s complexity. In this example, the DQ has three steps – proposal evaluations, risk analysis and setting up tests, which sounds problematic with a huge amount of work, but with proper setup, it is manageable.
In the first step of DQ, the user has to check if the supplier meets the requirements described in URS. Needless to say, if a supplier cannot meet all requirements, talk to them and find acceptable solutions for both or choose more appropriate supplier/solution. If you appended URS with FS as proposed in this article, a major part of the DQ can be done by commenting back to the supplier’s comments.
SENSUM TIP: Simply do DQ within the URS/FS document by justifying your decisions as in the example.
The second step of DQ is risk analysis and is started only after the first step is agreed between the user and the supplier. The outcome of risk analysis is risks and specifications, which need to be tested and addressed during qualifications.
SENSUM TIP: Risk analysis is a difficult task, especially if the technology is new for the user. Do not try to fabricate a possible risk for each URS point. Use experience and common sense. If risks are too hard to define for any reason, the supplier should be able to help you with risk analysis. The supplier knows the solution in-depth better than anyone.
The last step of DQ is setting up qualification tests for the verification phase of the V-model. The tests should check whether the supplier is providing everything as agreed and should address any risk that was above the risk threshold.
SENSUM TIP: Supplier’s IQ/OQ document will include tests for most of the required points and risks. Check those tests first before starting to set up any new tests. Also, try to justify general requirements and risks with functionality to simplify your qualification protocols and minimise redundant testing. As an example, let’s assume a risk: “A camera in the inspection system is not working.”. Do not make a special test to check, if a camera is installed, connected to power and is working. Assign the risk to a general test, such as “machine start-up”, which you will do anyway, and justify, that you could see live images on HMI after start-up, and therefore, the system has a functional camera. In another example, let us now assume a user requirement on audit trail: “All actions on the machine must be recorded in the audit trail.”. Don’t make a special test “check audit trail”. Try to assign the requirement to any operational test, where batch report with audit trail will be checked for any other reasons.
The final result of DQ is Traceability matrix, which links risks and requirements with tests.
SENSUM TIP: Traceability matrices are known for many things. To save the project team’s time is not one of those things. The challenge is to make connections between URS, risks and tests clear and as simple as possible. By experience, there will always be more URS points than risks in number. For that reason, assign URS points to risks and not vice versa. Some URS points might even go un-assigned, which will only indicate that un-assigned URS points are not risky for the project.
Verification phase: Finally, the IQ, OQ and PQ
When the specifications phase is finished and the supplier is ready for the installation, the verification phase begins. The user and supplier will follow IQ/OQ protocols and the user will conclude qualifications with PQ.
SENSUM TIP: User and supplier should agree on the exact protocol and scope of tests during DQ to minimise making up new tests during the qualification, which is risky for both parties.
IQ/OQ is typically done twice. First, it is done at supplier place as part of factory acceptance tests (FAT). During FAT, any changes to the system due to requirement changes (oh, those happen often) or due to possible deviations are not as expensive as later, when the system is outside of manufacturing facilities.
SENSUM TIP: FAT is usually the user’s first experience with the machine. Spend time on OQ as much as possible, because OQ consists of tests, where the machine is performing its job. It is hard to imagine a worse deviation as safety or functional deviation. However, IQ is still prerequisite for OQ, so try to get it done as quick as possible by only doing necessities and by skipping more administrative tests with “N/A at FAT” or “Not risky, to be tested at SAT” to get to OQ as fast as possible.
Second, IQ/OQ is repeated with the same products after final installation at the user’s site as part of site acceptance tests (SAT).
SENSUM TIP: Send the final user (operators from production) to FAT / SAT. If operators perform the tests, FAT and SAT become very efficient training at the same time. In addition, try to make operational tests from OQ feel like real operation by including tests for the set-up of hardware and software, tests for feeding/discharging, tests for long term operation, performance evaluation, waste generation evaluation, throughput checks and downtime tests, such as cleaning tests and assembly/disassembly tests. OQ experiences will be highly welcome when preparing for PQ.
Performance Qualification (PQ) is performed by the user after a successful SAT. The user should have prepared a Standard Operation Procedure (SOP) and follow it during the PQ. Products made/processed at successful PQ can be already used commercially.
SENSUM TIP: The supplier can help you optimise your SOP, which will be used for many years. Optimisation and modification at this early point will improve the success rate of PQ and will improve the success rate of all later runs.
Download URS, Risk analysis with traceability matrix, IQ, OQ examples from the link in the company logo at the beginning of the article.


