
The use of mRNA in therapeutics is growing ever wider following its success in Moderna’s and BioNTech’s Covid-19 vaccine programmes. Offering enormous potential to transform patient care through personalised medicine, the next big step for researchers and scientists is combining in vivo – within the body – mRNA delivery with gene editing technology, like CRISPR. This approach could make it possible to cure mutated, disease-causing genes directly inside the patient and reduce the overall costs associated with current ex vivo technologies.
Earlier this year, in a historic medical breakthrough, a child with a rare metabolic disorder was treated successfully with mRNA-based gene-editing technology. This event has sparked mRNA’s next phase, says Dr Cody Palumbo, a Senior Application Scientist at TriLink BioTechnologies, a biotechnology company that has been refining and innovating in mRNA design and manufacturing workflows for 30 years.

According to healthcare market intelligence firm GlobalData, 458 mRNA-based gene-editing drugs were being tested in clinical trials as of 30 October 2025. Of these, 44 drugs were in Phase I and Phase II and more than 50% were in discovery and pre-clinical phases.
For biotech and pharma companies, this opens huge opportunities to expand mRNA into new therapeutic areas and applications outside of infectious disease vaccines. “But to fulfil the potential of this new field of medicine, there are mountains of manufacturing challenges to circumvent,” Dr Palumbo adds. “These include achieving precise mRNA design and high-quality production for GMP and whether at the start of a programme or in late-stage phase, specialised formulation know-how is an imperative.”
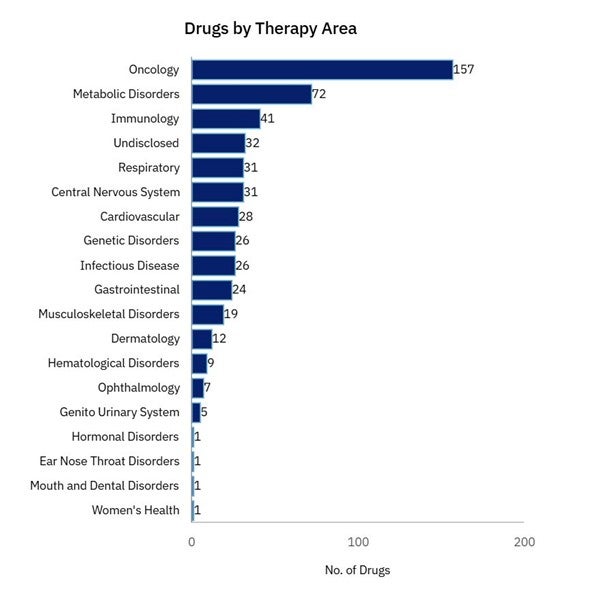
GlobalData Drugs Database: mRNA-based gene-editing drug assets in clinical trials by therapeutic area
So, why is mRNA the ideal platform for these lifechanging treatments – and what are some of the key obstacles be in getting these drugs to market?
In this Q&A, Dr Palumbo shares his views.
What is in vivo cell therapy – and how does it differ from previous gene-editing treatments?
Dr Palumbo: In vivo cell therapy involves genetically engineering cells directly within the patient’s body. This differs from ex vivo approaches, such as Vertex Pharmaceuticals’ Casgevy for sickle cell disease, where cells are modified outside the body and then infused back in.
Casgevy is the first therapy using the CRISPR-Cas9 gene-editing system to get approval from the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA). Widely considered to be groundbreaking, GlobalData projections show it could reach blockbuster status by 2030.
The current ex vivo (or outside the body) cell editing workflow is highly complex, requiring many coordinated steps before manufacturing can begin, and the costs remain significant. These challenges limit the scalability of cell and gene therapies and are accelerating the shift toward in vivo delivery.
Clinical trials of in vivo cell and gene (CGT) therapies have traditionally used a viral vector or DNA plasmid as a carrier. Viral vectors generally offer higher efficiency but pose greater safety risks. For one, as they enter the cell nucleus, there is the possibility that they will integrate into the host genomic DNA with unintended integration effects such as insertional mutagenesis or activating undesired genes. Other risks include unintended immune responses, toxicity from the vector or transgene, and genomic instability from unintended cuts in DNA. DNA plasmids are considered safer regarding immunogenicity and integration but have limitations in delivery efficiency.
What makes mRNA the ideal platform for in vivo editing over other traditionally used methods?
Dr Palumbo: The mRNA transfection process is simpler than DNA transfection. Because mRNA is directly delivered and expressed in the cytoplasm, after delivery it can immediately be translated into a protein. As the mRNA is also transient by nature, meaning the mRNA is only expressed for a short period of time, and then is naturally degraded. Lastly, it is rapidly gaining prominence because it can leverage existing non-viral lipid nanoparticles (LNPs) as the carrier.
The landmark case published this year of mRNA-based in vivo personalised medicine administered successfully to baby KJ provides proof of concept that bespoke therapies can be a clinical strategy.
Baby KJ was born with severe carbamoyl phosphate synthetase 1 (CPS1) deficiency, a rare metabolic disorder where the body cannot effectively digest protein. This leads to ammonia building up to toxic levels, which can cause organ damage, particularly in the brain and the liver and can be life threatening. In six months, a team of academic and industry scientists had developed a customised CRISPR gene editing therapy delivered via lipid nanoparticles to the liver with the aim of correcting KJ’s faulty enzyme, and early results are incredibly promising. This case shows how swiftly mRNA can be manufactured. Within weeks, clinical batches can be generated after the availability of a sequence encoding the target of interest.
What applications will we see cell and gene therapy targeting first?
Dr Palumbo: Oncology is the dominant therapeutic area the industry is focused on, and then rare diseases and blood disorders, but all therapeutics areas are being actively explored. Because of my industry background, I am personally excited by the progress in early clinical trials that aim to eliminate Type 1 Diabetes. Between 1.5 and 2 million people in the US alone live with T1D, an autoimmune disease where the immune system attacks insulin-producing cells in the pancreas.
That represents huge potential to help people. The FDA’s Center for Biologics Evaluation and Research (CBER) has projected approving between 10 and 20 novel cell and gene therapies annually from 2025. The US regulator is committed to supporting this new field of medicine, introducing new designations to expedite clinical trials in this space.
What are the biggest challenges with regard to developing mRNA-based cell and gene therapy?
Dr Palumbo: mRNA is only a single component in a multi-component manufacturing and then wrapped up in a package that will constitute the therapy product. It’s technically difficult, logistically difficult and challenging from a regulatory perspective. Having been at the forefront of innovation of mRNA design and manufacturing workflows for decades, we are well equipped to support our partners efficiently through the whole process.
How specifically can TriLink Biotechnologies help the biopharma industry with regard to mRNA design and workflows?
Dr Palumbo: TriLink provides an end-to-end solution for biopharma partners, supporting programmes from early screening through all phases of clinical development and into GMP manufacturing. By placing innovation at the core of our business, we give clients access to products and expertise that are not available elsewhere.
For example, we have developed a novel polymerase enzyme to reduce double-stranded RNA, an artefact of T7 polymerase. Additionally, we have produced CleanCap® capping technology, the first to produce a Cap1 structure in a single co-transcriptional reaction, requiring only one bioreactor and purification step. Our latest cap analog, CleanCap® M6, which combines a 3’OMe modification on the m7-Guanosine with an additional methyl on the +1 Adenosine, optimises mRNA potency and achieves the highest protein expression of any CleanCap® variant.
In addition, TriLink’s new AI platform evaluates sequences for manufacturability at the discovery stage, further saving significant time and resources. Layered with decades of process optimization experience, this approach ensures finely tuned scale-up and consistent, high-quality mRNA production. Partners can provide a sequence and receive high-quality, ready-to-use mRNA.
To perfectly complement this mRNA, TriLink also offers custom guide RNA (gRNA) design and synthesis, acting as a one-stop shop for all the components needed for CGT workflows. Our gRNAs are produced with the highest purity standards, making them ideally suited for sensitive CGT applications where performance and reproducibility are critical.
The purity of a gRNA candidate in CRISPR is key to the success of the treatment. Hear from Dr Columbo in this webinar how to ensure gRNA quality across research, preclinical, and GMP stages.


