
In the past few years, many drugs intended to be sterile have been recalled due to lack of sterility assurance1. This is because non-sterile drugs could result in site specific infections as well as serious systemic infections which may be life-threatening. In addition, Container Closure Integrity (CCI) testing has been a growing concern in pharmaceutical packaging due to regulatory guidance to use an appropriate CCI test in lieu of sterility testing. In terms of stability, lack of CCI could result in oxidation, hydrolysis or loss of vacuum which may have a significant impact on drug efficacy and safety.
Based on a deep understanding of the challenges and requirements of the pharmaceutical industry, West Pharmaceuticals Services, Inc. adopted Quality by Design (QbD) principles in its NovaPure components to help mitigate the impact of the efficacy and purity of a drug product. The NovaPure product offering includes serum and lyophilization stoppers, syringe plungers, cartridge lined seals and plungers.
QbD is a systematic, science-based approach during development which emphasises product and process understanding, as well as process control. ICH Q8 (Pharmaceutical Development), ICH Q9 (Quality Risk Management), ICH Q10 (Pharmaceutical Quality Systems), ICH Q12 (Lifecycle Management) were reviewed and applied. It begins with pre-defined objectives. There is no reliance on specifications and testing to assure product quality; rather quality is built into products.
West NovaPure specifications have been established by defining Quality Target Product Profiles (QTPPs) for the components, as well as the related Critical Quality Attributes (CQAs) defined to meet QTPPs. Having a solid scientific understanding of the manufacturing process and the impact of the design of the items, West Pharmaceuticals were able to achieve the target for stringent part-to-part variation and high cosmetic quality that may minimise the risk of CCI directly (Table 1).
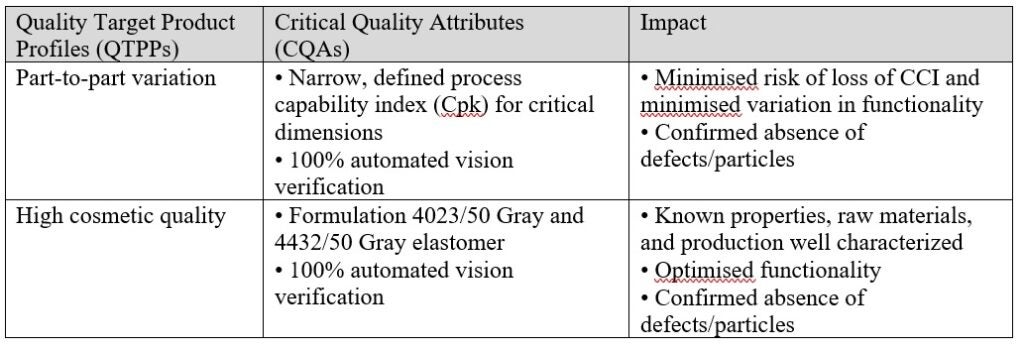
For NovaPure components, Process Capability Index (CpK) values and Parts Per Million (ppm) are used for release. The Cpk of the dimensions of NovaPure components (e.g., flange diameter, rib diameter, plug diameter, flange thickness and overall height that may lead to failures on CCI) should be greater or equal to 1.33.
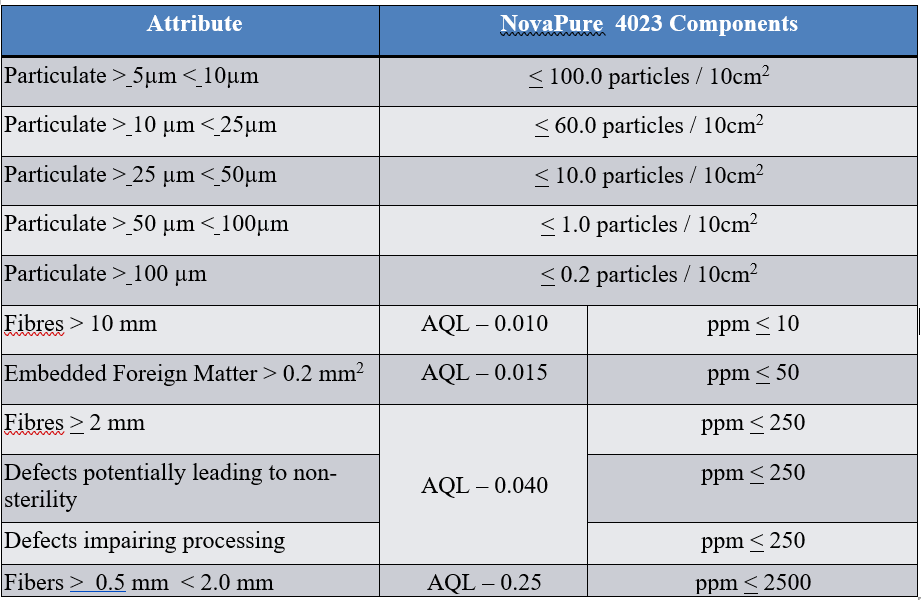
Visual defects of rubber closures are critical for CCI failure as they have the potential to affect integrity. For example, a fibre in the stopper or non-fill of land seal part of the stopper may lead to CCI issues. West’s 100% automated vision inspection provides consistency and control of critical quality shortcomings of the closure component. NovaPure components boost specifications that go beyond 100% automated vision inspection (Table 2).
- Not only should the dimensions and appearance of components be considered, but the consistency of physical properties over time too. Such quality attributes include the materials used, the hardness of elastomer, modulus, as well as elastomer viscoelasticity in particular.The viscoelasticity is an inherent feature of elastomers that may directly affect the quality of CCI over an extended duration. Thus the leading formula 4432/50G and 4023/50G were selected for NovaPure components.
The selection of Novapure components with the strictest specifications is an important part of any CCI control strategy. Ensuring good CCI is essential for drugs. NovaPure is an example of West’s commitment to the safe and efficient delivery of drug products to patients. For more details, please refer to West Technical Reports2345.
For more on how West can provide support, please contact an account manager or technical customer support (TCS) representative.
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts
- Technical Report 2013/150 NovaPure® 1mL Long 4023/50 Gray Plunger Container Closure Integrity Assessment.
- Technical Report 2018/192 1 mL Long and 1-3 mL NovaPure® Plungers 4023/50 Gray Container Closure Integrity Performance Evaluation in Glass Syringes via High Voltage Leak Detection.
- Technical Report 2020/219 Container Closure Integrity Testing on EU NovaPure® Closure Configurations for Ready Pack™ System with SCHOTT No Blowback Vials.
- Technical Report 2021/242 Container Closure Integrity Testing on NovaPure® and Daikyo Closure Configurations for Ready Pack™ System with Daikyo CZ 2 mL Vials.


