
Overview
Panbela Therapeutics Inc. (“Panbela”) is a biopharma company focused on developing small molecule therapeutics for oncology indications. Before partnering with Syngene, Panbela was producing its small molecule candidate SBP-101 for pancreatic cancer using a 15-synthetic step literature preparation.
When Panbela approached Syngene for the chemical development of its molecule, we recommended a chiral purity check of early batches to ensure compliance with FDA guidelines. The check showed up a chiral purity of only 85% – well below acceptable regulatory standards.
Syngene modified the synthesis approach by introducing a ‘reverse epoxidation’ strategy which prevented the formation of undesirable intermediates. This resulted in the production of SBP-101 with a chiral purity of 98% but using 17-synthetic steps.
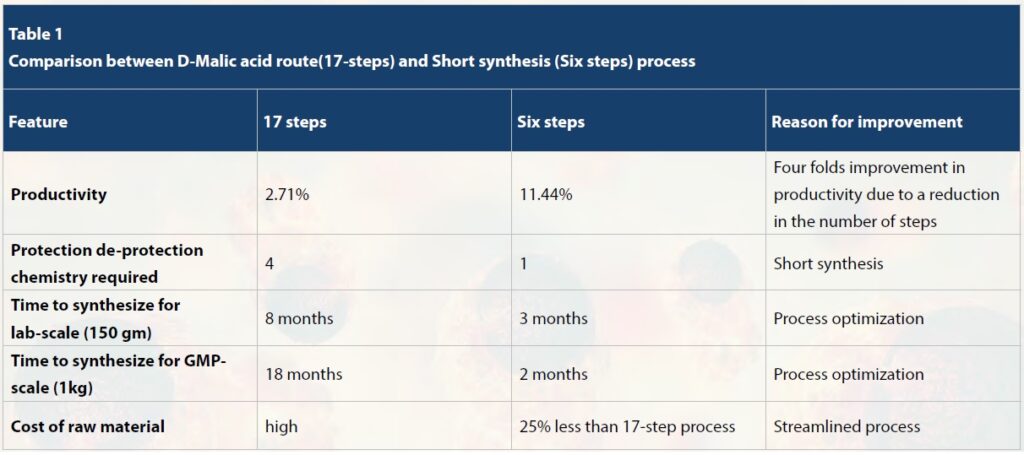
Panbela then tasked Syngene with reducing the number of reaction steps to achieve faster go-to-market of the drug while maintaining chiral purity. To achieve this, Syngene’s chemists began by first changing the starting material and imagining a new backbone building block for the molecule. This was followed by optimization of downstream chemistry and removal of column chromatography steps. This resulted in the synthesis of SBP-101 in just 6-steps while also improving the overall yield by fourfold from 2.71% to 11.44%.
Syngene went on to successfully demonstrate 4kg GMP manufacturing of SBP 101 for a Phase 1 monotherapy study. Panbela is now planning to proceed with commercial validation of the short synthesis process.
About the client
Panbela Therapeutics, Inc. is a clinical-stage biopharmaceutical company developing disruptive therapeutics for patients with urgent unmet medical needs. The company’s initial product candidate, SBP-101, is for the treatment of patients with metastatic pancreatic ductal adenocarcinoma, the most common type of pancreatic cancer. Panbela Therapeutics, Inc. is dedicated to treating patients with pancreatic cancer and is exploring SBP-101’s potential when used in combination with other agents for additional cancer indications.
The Challenge
Panbela, partnered with Syngene for the synthesis of SBP-101, a polyamine metabolic inhibitor used for the treatment of patients with metastatic pancreatic ductal adenocarcinoma.

Earlier, Panbela research team had explored a 15-step process to develop the small molecule using a literature preparation originally developed at the University of Florida. Later, Panbela partnered with Syngene for the chemical development of the same molecule (SBP-101).
Syngene’s chemical development team recommended conducting a chiral purity check of the product post-synthesis to ensure compliance with FDA guidelines.
The SBP-101 structure lacks a UV absorption signal as it consists of an aliphatic chain with two chiral centers. This makes the HPLC method development for determining chiral purity all the more challenging.
The Solution
Method development for chiral purity
Syngene’s chemical development team synthesized the RR, SS and RS isomers of SBP-101 and developed a new chiral method to determine the chiral purity of SBP-101. The chiral method also distinguished RR, SS and RS isomers. The chiral method was based on a two-step derivation which determined that early batches using a 15 step synthesis had only a chiral purity of 85%. This was below acceptable regulatory standards. In the 15-step synthesis, the chiral purity of the epoxide intermediate was less due to the formation of primary and secondary tosylates.
Syngene scientists modified the synthesis approach by introducing a ‘reverse epoxidation’ strategy which prevents the formation of the undesirable intermediates (primary and secondary tosylate) for achieving high chiral purity. The method was called as D-Malic acid route. This resulted in 17- synthetic steps (15+2 derivatization step) for SBP-101 production and met the client’s specification of more than 98% chiral purity.
Panbela then entered into a partnership with Syngene to streamline the production process by reducing the number of steps required for achieving high chiral purity.
Identification of simple starting material
As a first step, Syngene team replaced the existing raw material with (S) (-)4-chloro-3- hydroxy butyronitrile. It is a pharmaceutical starting material that is widely available, assuring the company of drug supply for long-term production.
Syngene then came up with the key synthetic step where the backbone of the molecule was constructed by alkylating a benzylic secondary amine with an aliphatic halide. Several reaction conditions were screened, and a common reagent−sodium bicarbonate NaHCO3 in ethanol was used for alkylation. This novel step is not reported in any literature and is now patented for the short synthesis of SBP-101.
Syngene team successfully optimized the downstream chemistry that involved cyano reduction, acetylation, Lithium aluminium hydride (LAH) reduction, and debenzylation. The 17-step procedure involved multiple de-protection and protection steps as compared to the new short synthesis, which was ‘atom economic’. The new method did not require any protection and de-protection measures.
The rigorous optimization studies resulted in a 6-step short synthesis method for SBP-101 production from the earlier, intensive 17-step method for synthesis.
Cost-effective reagent and reduction in process steps
Syngene replaced the hazardous LAH step with the more cost-effective Raney Nickel reagent. The column chromatography step was removed for acetylation and Raney Nickel reduction. Due to the reduction in the number of steps, the overall yield improved.
New technology developed
The process chemistry developed by Syngene scientists resulted in the filing of a patent (US 11,098,005 titled “METHODS FOR PRODUCING (6S,15S)-3,8,13,18- TETRAAZAICOSANE-6,15-DIOL”) for developing an anti-cancer drug. The advantage of this method is that it can be used to discover similar therapeutic small molecules.
Moving from lab-scale to GMP scale
Syngene produced 400 mg of SBP-101 through short synthesis while meeting chiral and chemical purity requirements (>98% chiral purity). Once Syngene had successfully demonstrated small-scale production of the molecule, Panbela requested a lab-scale synthesis of 50 g. Syngene delivered 150 g of the target molecule. The 6 step process had resulted in the overall yield improving four folds from 2.71% to 11.44%. As a part of the GMP process, 4.0 Kg of SBP-101 was manufactured through the new short synthesis and delivered to Panbela. This demonstrates Syngene’s ability to support large-scale manufacturing for different phases of clinical trials.
State-of-the-art technology used
HPLC-CAD was used for purity and analytical validation, while mass spectroscopy was used for checking the compound for purity. NMR was used for chiral purity analysis. The chiral method for SBP-101 (SS isomer) was developed by synthesizing relevant impurities (RR, SS and RS isomers of SBP-101).
Benefits of short synthesis
- Faster time-to-market due to simpler production process
- Final product which is pure and of high-quality
- Reduced lead time for product manufacturing
- Economical starting material and chemical reagents
- Scalable, efficient, and cost-effective manufacturing route for future commercialization
- Fast access to drug supply facilitating expansion into additional therapy areas
Conclusion
With Syngene successfully demonstrating GMP manufacturing of SBP 101, Panbela has given the go-ahead for commercial validation of the procedure in order to proceed with clinical trials. Commercial validation is pending review with FDA.
Based on Syngene scientists’ invention of a successful short synthesis of SBP-101, a US patent was filed by Panbela naming Syngene scientists as the inventors of this new production process.
Panbela has completed a Phase – I monotherapy study in previously-treated pancreatic patients, and a Phase – I study in first line metastatic patients in combination with gemcitabine and nab-paclitaxel.

This project brings to the fore, Syngene’s expertise in production processes for an investigational new product. The company has now further extended its partnership with Syngene till 2030. So far, Syngene has worked on more than 30 projects related to drug substance development for Panbela.
We look forward to more such partnerships where we can help clients like Panbela break new ground in developing innovative therapies and treatment for patients globally.
Testimonial
“Syngene streamlined the production process of Panbela’s pancreatic cancer drug, SBP-101 while ensuring high chiral purity of 98% during GMP manufacturing. In recognition of the successful development of SBP-101 short synthesis by Syngene scientists, Panbela filed a US patent application naming Syngene scientists as the inventors of this new production method for SBP-101, This project undoubtedly brings to the fore, Syngene’s expertise and dedication in production processes for our investigational new product.”
Thomas X. Neenan, PhD – Chief Scientific Officer, Panbela Therapeutics Inc.


