
The journey from groundbreaking pharmaceutical research to widespread deployment of novel drug treatments is riddled with challenges. The recent success of mRNA vaccines – most notably in the context of the Covid-19 pandemic – has seen the technology’s promise flourish across a range of therapy areas. From cancer to infectious diseases, such therapies are becoming increasingly promising for ailments once believed to be intractable.
Whether in the realm of prophylactic vaccines or emerging gene-editing research, mRNA’s flexibility allows for customisation – one of its key advantages. The process begins with a DNA template, a precursor to synthesizing mRNAs by in vitro transcription. The approach to mRNA synthesis – along with 5′ capping, 3′ tailing, and modification of the bases – plays a crucial role in the efficient translation and potency of the mRNAs. On the delivery front, lipid nanoparticles (LNPs) have emerged as the dominant mechanism, ensuring stability and efficient cellular uptake of mRNAs.
Meanwhile exploration into alternative delivery mechanisms is continuing apace. Polymer nanoparticles and exosomes exhibit particular promise. Modified nucleotides have become a key focus in the mRNA world, with their ability to evade innate immune responses opening new avenues for research. Modified bases, such as N1-methylpseudouridine, enable mRNA to bypass overstimulation of the innate immune system, contributing to the overall success of mRNA therapies.
But as companies strive to replicate the success of mRNA vaccines beyond Covid treatments, the trials and tribulations inherent in ensuring quality control throughout the manufacturing process have become more apparent. One of the pivotal aspects of mRNA manufacturing, for example, is the generation of the 5′ cap, essential for mRNA functionality. Getting this right is not easy. Deployment of outdated technology to do so can see timelines lengthen and costs rise. Furthermore, the gap between demand for mRNA materials and their supply is starting to widen. The impact of the COVID-19 pandemic initially led to a slowdown in trials, but the momentum picked up again in 2021 and 2022, doubling in 2023. Encouraging results in early-stage melanoma have likely contributed to this resurgence.[1] Innovation will be crucial to help bridge the gap.
New technologies driving change
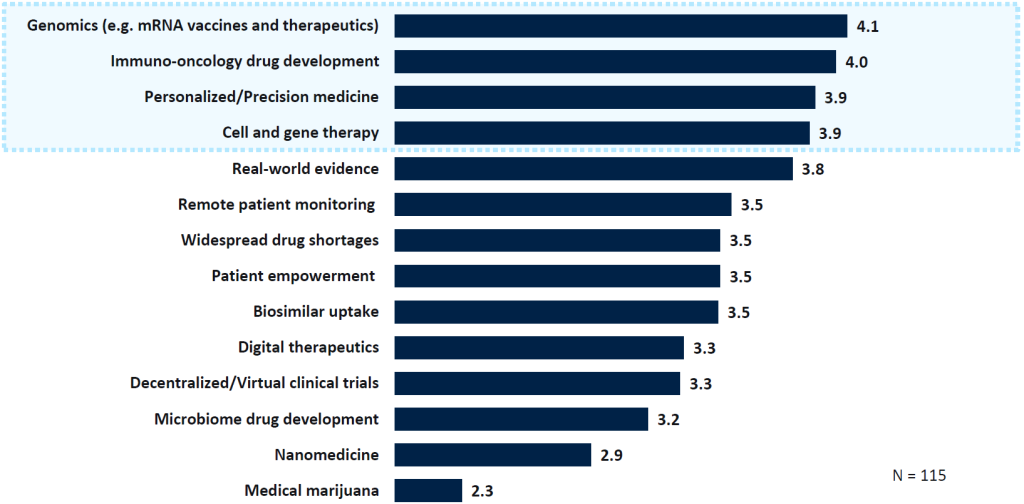
A survey conducted by GlobalData at the end of 2023, polling over 100 healthcare industry professionals, found genomics was regarded as the most potentially impactful pharmaceutical trend for the coming year. It was followed by immune-oncology drug development, personalised medicine and cell and gene therapies. These trends, building upon previous years’ patterns, underscore the transformative influence of genomics and advanced therapies in shaping the future of medicine.
The rise of genomics places new demands on biomanufacturers. Working in unchartered territory, novel solutions are hitting the market all the time to refine quality control in rapidly evolving processes. Generating the 5′ cap is a standout example. The challenge lies in utilising synthetic caps that meet the demanding requirements of stability, expression and immunogenicity; hitting regulatory benchmarks to meet quality standards can be an intricate and costly process.
This is where innovations like patented CleanCap® technology, TriLink BioTechnologies’® market-leading capping solution, are making headway. It deploys a co-transcriptional approach to ramp up the efficiency of conventional mRNA capping strategies. In doing so, the technology shortens manufacturing processes, reduces overall costs, and enables biomanufacturers to meet product development deadlines while cracking down on costs. As Jason Downing, Senior Product Manager from TriLink, explains: “Determining your mRNA design and process early on in development will pay dividends. It may be harder to switch sequence, process, or purification as you venture further down the drug development path. CleanCap® cap analogs can be used at any scale with the same streamlined one-pot process allowing for simple comparability between scales or phases.”
TriLink’s CleanCap® technology has already left a notable mark on the biopharmaceutical landscape in the rapid development of Pfizer and BioNTech’s COVID-19 vaccines.[2] Co-transcriptional capping played a crucial role in expediting the market introduction of these lifesaving vaccines. Comparing CleanCap® technology with traditional mRNA production methods highlights its advantages in speed, cost, and quality control. An enzymatic capping strategy involves two bioreactor reactions – the first to synthesize mRNA and the second to cap it. This often requires additional purifications, reagents and enzymes to produce the natural Cap-1 structure, with expected yield values between 50 to 70%. In contrast, CleanCap® technology’s one-pot process produces the Cap-1 structure in a single co-transcriptional reaction, eliminating the need for multiple steps, reagents and enzymes. This streamlined approach can result in capping efficiency ≥95%.
What’s next?
As of December 2023, there were 12 DNA- or mRNA-based oncology drugs either approved or in pre-registration.[3] An additional 363 are in Phase II or III development globally. This surge in development reflects a significant year-over-year uptick; in the prior period, only 40 drugs were marketed across all therapy areas.
Advancing the most technically demanding aspects of mRNA production, like synthetic cap manufacturing, holds the key to the technology continuing to scale up. In comparison to other capping methods, CleanCap® technology can potentially cut mRNA therapeutic production processes by a week and reduce overall manufacturing costs by as much as 40%. This advantage stems from the ability of co-transcriptional caps to simplify synthesis reactions, making development requirements more straightforward. If the potential of mRNA therapy is to be realised, innovations like CleanCap® analogues have a fundamental role to play.
Trailblazing biomanufacturers cannot stay ahead of the pack unless every aspect of their production is optimised. By supporting rapid production times for mRNA, while also facilitating high yields of unprecedented quality, CleanCap® technology is an example of innovation that helps to do just this. As the creators of CleanCap® technology – and having prefigured and pioneered the rise of mRNA therapies – TriLink BioTechnologies® is home to unrivalled expertise in raw materials for nucleic acid therapeutics, mRNA manufacturing, analytical services and quality control. Download the whitepaper on this page to find out how they are helping biomanufacturing firms big and small transform their efficiency – and how they could help you too.
[1] GlobalData Thematic Intelligence, “The State of the Biopharmaceutical Industry: 2024 edition”, page 25
[2] https://pubmed.ncbi.nlm.nih.gov/32998157/
[3] GlobalData Thematic Intelligence, “The State of the Biopharmaceutical Industry: 2024 edition”, page 24


