
Multiple headspace extraction gas chromatography (MHE GC) is an analytical technique that is used to calibrate a headspace GC system where all other ‘normal’ approaches have failed.
In its simplest form, MHE mimics the fully exhaustive extraction of targeted volatile components of a sample, with quantitation obtained by comparison with a totally vapourised (i.e. single phase) external standard of the targeted analytes. When MHE is applied, each sample or standard vial is incubated and injected into the GC several times, with the pressure in the vials being vented to atmospheric pressure after each injection and before subsequent incubation cycles. MHE plots of log area versus injection number are constructed and extrapolated to zero, and enclosed areas are directly compared to obtain quantitation.
MHE, when applied to a sample or standard placed in a HS vial, gives a declining peak area with each repeated incubation and injection cycle. Assuming the amount of sample entering the GC instrument is trivial relative to the vial content, when the vial is vented to atmosphere post-injection, half of whatever is in the gas phase should be vented. If a small volume, say 20µL of a standard, is placed in a vial and completely vaporised, the peak area for analytes will decline as fast as is possible (i.e. there is only one phase with no partitioning involved). Any MHE plot for a sample where there is phase partitioning will result in a lower gradient. In practice, it’s not required to continue the incubation and injection cycles for sample and standard until the vials are completely exhausted. Normally, four cycles are enough to establish accurate MHE plots. A plot of log area versus injection number must be linear for the MHE analysis to be valid.
The example below shows MHE plots obtained for the analysis of styrene monomer in a sample of polystyrene.1
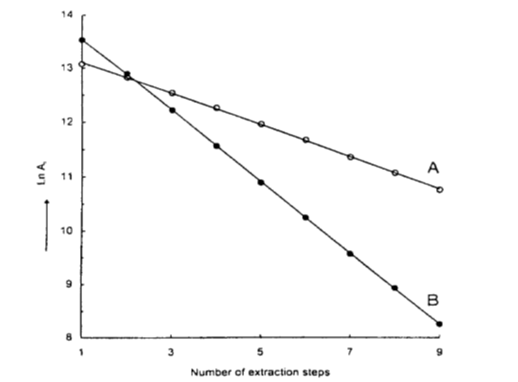
The MHE plot labelled B was obtained for the standard vial containing a totally vaporised small volume of styrene standard; A is the plot obtained for the sample polystyrene. As expected, the gradient of B is greater than the sample plot since no partitioning is involved.
MHE is not applicable to analytes that are highly soluble in the matrix under the conditions used. If 99.9% of the analyte is in the matrix, MHE will result in near horizontal plots. If the MHE plot is non-linear, this probably indicates equilibrium conditions are not being achieved in the vial. This may occur for several reasons, the most obvious being an incubation time that may be too short, coupled with slow migration of an analyte from a solid sample matrix into the headspace of the sample vial.
The concentration of the standard preparation is arranged so the required mass of calibration substance is contained in a small volume (i.e. 10µL – 20µL) of solvent, which will be vapourised when the standard vial is incubated. It’s required to add the same small volume of solvent to the sample vial. The addition of solvent to the sample vial equalises pressures in standard and sample vials, while the higher boiling analytical solvent helps displace analyte – especially if active sites are present in the solid sample. A high boiling solvent can form a thin liquid film on the surface of the sample, which aids extraction of analytes. This is called surface modification.1
The use of MHE can reduce the time and effort required for the determination of total lifetime exposure to ethylene oxide sterilisation residuals from permanent contact medical devices required for compliance to ISO 10993-7. Instead of having to complete a time-consuming and expensive cycle of liquid extractions followed by analysis, determination can be obtained from a single sample preparation in a matter of hours. And, unlike solvent extractions, the MHE analysis is fully automated apart from adding the sample or standard into a headspace vial.
For many years, Butterworth Laboratories has been applying MHE to solve the problem posed by insoluble sample types. Applications have included: the analysis of ethanol solvent from printed plastic films; identification and semi-quantitation of residual solvents in plastic film adhesive tape using MHE GC with mass spectroscopy detection; residual vinyl chloride monomer in PVC plastics; residual solvents in a variety of insoluble pharmaceutical materials; and the determination of ethylene oxide sterilisation residues in surgical sutures.
The applications for MHE are set to continue to increase since the technique can provide fully quantitative analytical results not readily possible by any other means of exhaustive extraction.
Author Biography

Frank Judge BSc (Hons) CChem MRSC MChromSoc CSci
Consultant Chemist, Chromatography
Frank started his career at Kings and Co as a Senior Technician before joining Berridge Environmental Labs as Organic Analysis Team Leader in 1990. After a short spell with Pharmaco LSR in its Department of Aquatic Toxicology Studies, he joined the Chromatography Department of Butterworth Laboratories in 1994 and has progressed through various roles to his current position. Frank has spoken at JPAG meetings on Organic Volatile Impurities analysis and has been trained in preparing expert witness statements.
[1] Static Headspace Gas-Chromatography: Theory and Practice by Bruno Kolb and Leslie S. Ettre, April 2006.


