
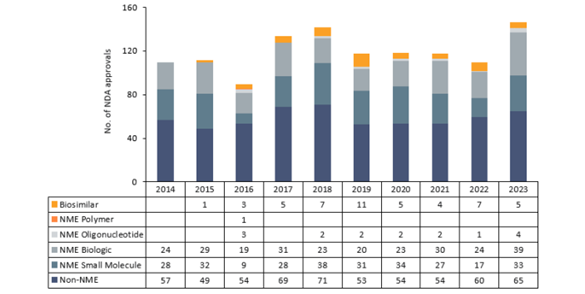
In late 2023, the first CRISPR-based gene therapy for sickle cell disease patients Casgevy, received the seal of approval from the UK’s NHS, followed quickly by approval from the FDA[i]. In general, 2023 saw a jump in the number of FDA approvals, with ~145 small-molecule drugs and biologics (including new molecular entities and new formulations of existing drugs) approved — a ~21% increase compared to the average for 2018-2022. Of those, 7 were new cell or gene therapies, the highest number approved in any year since 2014[ii].
However, gene therapies have historically been complex and resource-intensive to produce, with lengthy manufacturing timelines and limited production capabilities, owing partly to a scarcity of qualified staff and appropriate facilities. As the number of gene therapy approvals continues to rise, the pressure on manufacturing capabilities will intensify. The industry’s future will likely see a greater emphasis on collaboration among pharmaceutical companies and contract development and manufacturing organizations (CDMOs), while technology providers will strive to overcome existing challenges and enhance the scalability of gene therapies.
Addressing key challenges
Gene therapy manufacturing currently faces numerous challenges, including limited stable producer cell line technologies and insufficient analytics, as well as high capital investment requirements, long development timelines, and expensive raw materials.
During a recent webinar, Undoing the dogma: A changing ethos in AAV gene therapy manufacturing, leading experts came together to dissect the current challenges and future opportunities in viral vector production. Featuring panelists from industry, academia, and technology providers, the discussion offered a comprehensive look at the evolving landscape of gene therapy manufacturing. Adeno-associated virus (AAV) is a highly versatile viral vector that can be engineered for very specific functionality in gene therapy applications and is the most widely used virus for manufacturing gene therapies.
Dr. Nathalie Clément is Senior Vice President of Vector Development at Siren Biotechnology. Clément says there is a need for a more scientific, systematic approach to understanding and optimizing AAV manufacturing processes. “There is still a lot to do as a developer and manufacturer,” she says. “I believe we need to focus on three different levels. The first is to keep focusing on the upstream; you need to keep improving your yield without [decreasing] the quality of your AAV drug product. On the downstream side, even though we have powerful tools right now, I still think we can improve on that and make them even more specific (and) increase their capacity.
“The second is looking down the line a little bit. We need to start simplifying processes if we really want to have something robust, consistent, affordable, and fast. We know that each time you add a step, you lose vector. Simplification is something that we should see more of — making everything simpler and faster. Finally, in the long term, we must think about automation and standardization. Automation is going to be the way to go for consistency and affordability.”
Boosting upstream yields and enhancing process consistency
Sybil Danby, General Manager of Cell Lines at Cytiva, works with customers to enable process technologies that make viral vector production more effective. Danby observes that boosting upstream yields requires a holistic approach that combines technological innovation with deeper biological insights to consistently improve vector production efficiency.
Danby sees stable producer cell lines as a transformative technology for AAV manufacturing, providing consistency and a more stable foundation for process improvements, while also reducing batch-to-batch variability.
“We’ve made really great advancements over the past, say, four to six years or so,” she says. “If we consider the roller bottles, flatware, and ultracentrifugation as first generation, where we are now is the second generation. But what does that third generation look like? We can talk about all these great process improvements, but it’s not always easy to make changes.”
Simplifying downstream steps
Modular manufacturing is a key solution for pharmaceutical companies to refine and future-proof their production models. This method uses standardized, off-the-shelf functional modules, which can enhance operational efficiency and support compliance with good manufacturing practices (GMP). It allows for customization of production lines without extensive infrastructure modifications, allowing them to quickly adapt their manufacturing capabilities to meet individual vaccine demands.
Additionally, advancements in AI and machine learning are revolutionizing the manufacturing landscape, accelerating target identification and streamlining gene editing processes, making the development of therapies faster and more efficient.
Opportunities, innovation, and regulation
In the quest for higher-quality and scalable AAV production, opportunities center on transforming current manufacturing limitations through strategic innovations. Historically, viral vector production relied on techniques like roller-bottle cultures and ultracentrifugation, which were time-consuming, low-yielding, and challenging to scale.
Today, the industry is increasingly adopting suspension bioreactor technologies, which provide a more scalable and efficient means of producing AAV vectors. These systems allow for higher cell densities and better nutrient distribution, which can lead to exponentially higher vector yields compared to traditional adherent culture methods. Specialized chromatography resins tailored for AAV purification improve the purity and yield of the final product by enabling more effective separation of the desired viral particles from contaminants.
This convergence of advanced technologies, including improved transfection methodologies and enhanced analytical processes, is driving a paradigm shift in gene therapy manufacturing. This evolution not only addresses current challenges but also positions the industry for future growth and innovation. These innovations address challenges such as reducing production costs, increasing vector quality, enabling larger-scale manufacturing, and accelerating timelines from development to clinical production.
The importance of working with regulators
Danby notes the importance of regulatory authorities in helping to enable the adoption of such innovative technologies. Regulators are increasingly supportive with ongoing dialogue between them and industry, ensuring that regulatory guidance is evolving to support innovative manufacturing technologies.
In 2024, for example, the FDA released draft guidance on a program to advise technology providers on how their platform technologies could be incorporated into drug development processes[iii]. In the EU, the European Medicines Agency (EMA) offers the PRIME scheme[iv], which similarly aims to help expedite review for medicines targeting unmet medical needs, rather than specifically designating platform technologies.
The role of CDMOs
CDMOs already play a crucial role in the gene therapy landscape, particularly due to the complexity and high costs associated with the production of gene therapies. The production of gene therapy products is technically complex, requiring substantial expertise and capital investment, and many companies rely on CDMOs to manufacture their product candidates.
The ability of CDMOs to contribute to cost reduction is vital for making these therapies more accessible. Additionally, as the market matures, CDMOs are expected to enhance their capabilities to meet rising demands, which includes developing larger facilities and increasing production capacity. Their role in ensuring that gene therapies can be produced at scale, with the quality and cost-effectiveness required to make them viable treatment options, is seen as crucial.
Overall, CDMOs play a vital role in enabling AAV manufacturing but are not necessarily the primary drivers of transformative innovations, says Nathalie Clément: “I think one of their roles is to enable bringing innovations to the forefront, so that their clients can benefit from them.”
Conclusion
Gene therapy is revolutionizing patient care, but challenges in large-scale production, including standardization, purification, and low yields, are hindering progress. As gene therapies move from rare disease treatments to broader applications, new manufacturing technologies are becoming ever more essential in making therapies more accessible and economically viable. Emerging technologies like fully stable producer cell lines and advanced analytics offer the potential to accelerate breakthroughs in the coming years.
Cytiva addresses these issues with its cell line development solutions, such as the ELEVECTA™ platform, which offers plasmid- and helper virus-free technology. They also provide flexible media and bioreactors for various cell types and modern chromatography resins for efficient separation. Cytiva’s systems aim to streamline production from development to manufacturing, enabling researchers to focus on innovation.
For further information on how Cytiva can help you in the quest for higher quality and scalable AAV production, download the free paper below.
[i] https://www.pharmaceutical-technology.com/news/first-gene-therapies-for-sickle-cell-disease-secure-fda-approval/
[ii] GlobalData: New Drug Approvals and Their Contract Manufacture: 2024 Edition, April 2024
[iii] https://www.fda.gov/regulatory-information/search-fda-guidance-documents/platform-technology-designation-program-drug-development
[iv] https://www.ema.europa.eu/en/human-regulatory-overview/research-development/prime-priority-medicines



