US-based clinical-stage biotechnology firm Advaxis has received orphan drug designation from the US Food and Drug Administration (FDA) for its lead drug candidate ADXS-HPV to treat human papillomavirus (HPV) associated head and neck cancer patients.
The FDA orphan drug designation entitles Advaxis to clinical protocol assistance with the FDA, as well as federal grants, tax credits, and potentially a seven-year market exclusivity period.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company said that patients with head and neck cancer have limited treatment options and it intends to improve survival by developing ADXS-HPV for this indication.
Following orphan drug status, the company intends to start an additional Phase I/II study in early stage head and neck cancer for ADXS-HPV with a nationally recognised centre of excellence.
The company is currently conducting a Phase I study that involves investigating the safety and efficacy of ADXS-HPV in patients presenting with HPV related oropharynx cancer and who have been treated with surgery, radiotherapy, and/or chemotherapy, alone or in combination.
The Phase I trial is being sponsored by the UK’s University of Liverpool and Aintree University Hospitals NHS Foundation Trust.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAdvaxis president and chief executive officer Daniel O’Connor said: “Receiving orphan drug designation for ADXS-HPV in head and neck cancer is excellent news for a technology that may offer the potential to treat an indication with few therapy options, and, importantly, it helps define a clear path forward to registration.”
ADXS-HPV has also received FDA orphan drug designation for the treatment of HPV-associated anal cancer.
ADXS-HPV is an immunotherapy that is designed to target cells expressing the HPV gene E7 that is responsible for the transformation of infected cells into dysplastic and malignant tissues.
The company has designed ADXS-HPV to infect antigen-presenting cells and direct them to generate a powerful, cellular immune response to HPV E7.
Advaxis is developing ADXS-HPV for the treatment of cervical dysplasia (CIN), cervical cancer, cervical intraepithelial neoplasia, oropharyngeal carcinoma, and anal cancer, as well as head and neck cancers that result from HPV.
According to the company, ADXS-HPV can generate a robust immune response, break immune tolerance to cancer and produce an unusually strong and effective multi-level therapeutic immune response to existing cancer and other diseases.
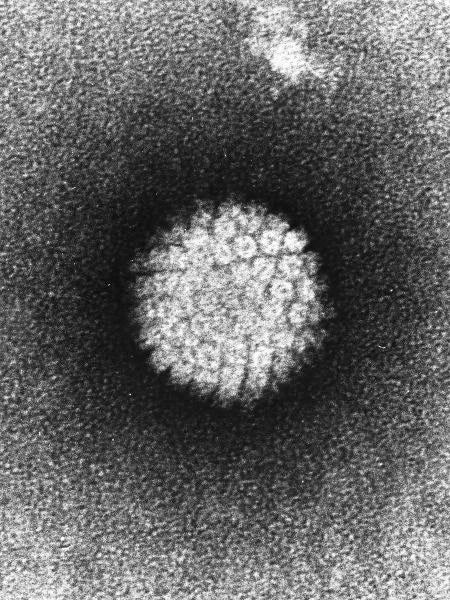
Image: Electron micrograph of a negatively stained human papilloma virus. Photo: courtesy of Patho.




