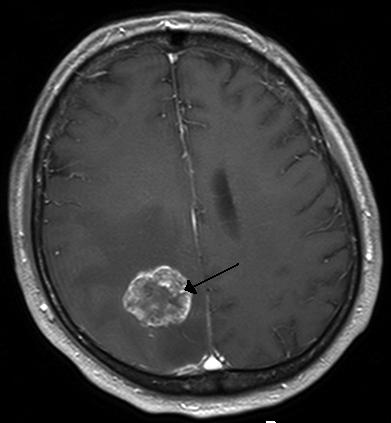
Two breast cancer drugs developed separately by GlaxoSmithKline and Roche helped treat the disease’s spread to the brain, a new study has shown.
When combined, GSK’s Tykerb (labpatinib) and Roche’s Xeloda (capecitabine) shrank brain tumours by 50% in 29 out of 45 participants, according to trial data published in the Lancet Oncology journal today.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Brain metastases occur in 30% to 50% of patients with metastatic HER2-positive breast cancer, and few systemic options are available to treat them.
Researchers in France and Austria said that most of these patients traditionally receive whole brain radiotherapy (WBRT), which can impair cognitive function.
Centre Leon Berard in Lyon, France, hospital practitioner and study lead author Dr Thomas Bachelot said; "Delaying such treatment for those patients is potentially a big advance."
"The patients had a median of 8.3 months before they needed radiotherapy, the study found."

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn this single-arm Phase II trial, eligible patients had HER2-positive metastatic breast cancer with brain metastases not previously treated with WBRT.
Of all 45 treated patients, 22 had grade 3 or grade 4 treatment-related adverse events, of which the most common were diarrhoea and hand-foot syndrome.
The study, sponsored by GSK and UNICANCER, showed that 14 patients had at least one severe adverse event and treatment was discontinued because of toxicity in four patients, but no toxic deaths occurred.
Given the side-effects, further study into the treatment is warranted, trial researchers have said.
Image: Brain metastases occur in 30 to 50% of patients with metastatic HER2-positive breast cancer. Image courtesy of Marvin 101.




