A biosimilar is a biotherapeutic that is clinically highly similar to an approved innovative product (reference product) in terms of active ingredients, and has no meaningful differences in efficacy and safety. They differ from generics: in complexity, the manufacturing processes and in the data needed to demonstrate similarity for approval. Biologics are potentially 1,000 times larger than a small molecule, far more structurally complex and they are manufactured in living cells, then extracted and purified, whereas small molecule drugs manufactured purely via chemical synthesis.
Biosimilars will assist healthcare systems globally to ensure that more patients than ever before have access to safe, effective and affordable treatments, often with better safety profiles than generic treatment options.
Biosimilar incentives
Considerable financial incentives exist for the development of biosimilars, their reference products tend to generate blockbuster revenues. Biosimilars can be 20–40% cheaper than the original biologic and therefore can attract considerable sales.
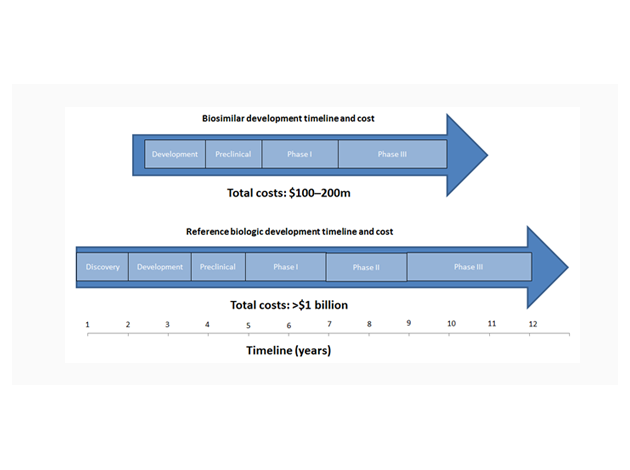
The developmental process for biosimilars is more streamlined than that of other molecule types, meaning the risk of attrition and cost (relative to the reference product) is reduced. Biosimilars do not need to be taken through the Discovery stage, as the therapeutic target of the original biologic is already known. Phase II is also not required as the dosage, efficacy and side effects of the biosimilar are the same as for the original product.

Challenges and complications
There are manufacturing challenges for biosimilars, primarily arising due to biologics being far more structurally complex and more difficult to characterize, produce, and reproduce than most small-molecule drugs. Even small variations in the manufacturing process can potentially alter the medicine’s safety and efficacy. The FDA must approve even minor changes in the production process. Achieving a sufficiently uniform product is difficult and costly even in different batches of the same product.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThere is uncertainty over legal factors concerning patents and trade secrets. The lack of automatic substitution and interchangeability with the original biologic has acted as a barrier to biosimilar development.
An approved biosimilar may enjoy a large market share for only a short time or perhaps not at all, because of the successful entry of another biosimilar or biobetter. There are fewer than 20 countries that have a defined regulatory pathway to market for biosimilars, which complicates the treatments development.
A positive outlook
There are numerous patent expiries that will occur for key biologic medicines in the next several years, which will increase the number of biosimilars being developed and marketed. With a few biosimilars such as Remsima, Benepali and Basaglar already marketed after post-2011 approvals.