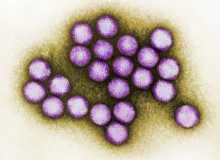
When, in late 2011, news became public that researchers had found a way to block adenoviruses by using plasma to damage the virus before it reached the host cells, excitement ran high. Pharmaceutical and specialist magazines were talking about a potential new cure in the fight against HIV, SARS, hepatitis and even the common cold. Never before had scientists been able to create plasma which could be applied to any kind of heat-sensitive materials, such as the human skin.
Published in the Journal of Physics D: Applied Physics, the study ‘Effects of cold atmospheric plasmas on adenoviruses in solution’, suggested that plasma – the fourth state of matter after solids, liquids and gases – was able to inactivate practically all highly contagious adenoviruses in a period of just 240 seconds.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
While the study results will almost certainly revolutionise the prevention of hospital-induced infections such as MRSA and perhaps noroviruses in hospitals and other healthcare organisations, it also holds the promise to transform pharmaceutical drug development in the far future.
Here, one of the study’s main researchers Julia Zimmermann, Max-Planck-Institut für extraterrestrische Physik research associate, gives some insight how plasma could change drug discovery and treatment of some of the most dangerous illnesses known to man.
Elisabeth Fischer: How is cold atmospheric plasma (CAP) produced in a laboratory?
Julia Zimmermann: It’s actually quite easy: you have lightning on earth, which partially ionises the surrounding air. That is the plasma. In the lab we’re creating tiny mini-lightning.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataWe have a metal surface where we place some sort of insulator, for example Teflon. On this Teflon-sheet we put a mesh grid and then stamp it all together in to a sandwich, so that there are no gaps of air in between.
Then we apply high voltage to the metal plate with a circular frequency and as a result we get mini-lightning on one side of the mash grid. With this mini-lightning we ionise the surrounding air.
This CAP device was used for the study with the adenoviruses, but we also have other plasma-devices which use argon to ignite the plasma. These are currently used in clinical studies on humans for the treatment of chronic wounds.
EF: Why did you choose to treat adenoviruses with the plasma?
JZ: Adenoviruses are one of the most resistant viruses and we had access to a lab with expertise in the field. These two facts combined were quite beneficial, because of course we wanted to research a more resistant virus than for instance HIV, which is not very resistant. We wanted to look at a more resistant virus against normal infectants.
EF: What happened when you exposed the adenoviruses to the plasma?
JZ: Unfortunately, we don’t exactly know what happened to the virus, we only know that it got inactivated. The exact mechanisms of what plasma does to different microorganisms are still unknown.
Plasma consists of a lot of reactive species and if you produce it in air, which mainly contains oxygen and nitrogen, you mainly produce oxygen and nitrogen species such as ozone, NO or NO2.
If you look at these components on their own, to some extent they’re all toxic for the virus but they would not be sufficient to kill it on their own. But combined into this plasma-cocktail, a lot of these active species seem to be quite effective against the adenovirus. But what exactly happens on the biological side or what biological mechanisms are involved – we don’t know.
Finding out what mechanisms are involved to kill the virus is actually part of our current research. It’s going to be very difficult to find out because plasma consists of so many things, and every individual part of the plasma, every single species, has some kind of effect on the virus or the bacteria.
If you look at the single species you’ll have to find out how much they contribute and what they do exactly. I believe it’s not one single mechanism but several mechanisms together.
EF: How would patient treatment with cold atmospheric plasma look like?
JZ: Before we can actually treat patients with plasma, we need to conduct more clinical research. We’ve done a Phase I study with a plasma device, which is a large device where the plasma is ignited with argon. We’ve set this up in two clinics in Germany, in Munich and Regensburg, and we’re now in a Phase II study where the plasma is used as an add-on therapy to treat chronic wounds. If you have diabetes you often have wounds which don’t heal because of the bacteria in the lesion. If the bacteria are multiresistant, no antibiotic will help.
This is obviously a major problem so we’re doing an add-on therapy where patients get a standard therapy with antibiotics or different creams, and in addition they receive plasma treatment once a day for two minutes. We were able to show that plasma-treated wounds have a significantly higher germ reduction compared to the controls.
Furthermore, we were able to show the same effect in standardised wounds. We treated acute injuries, which derived from mesh skin grafts, which are usually very defined. We treated one part of the lesion with plasma and the other with standardised treatment methods. We could show that wound-healing is clearly improved with plasma. We’ve also conducted treatments on skin irritations, such as the Hailey-Hailey disease, and plasma seems to work against different secondary infections. The next thing we want to look at is the herpes virus.
EF: Some media reports have suggested that plasma could soon be used to combat viruses such as HIV. How would that work?
JZ: I wasn’t particularly happy to see these media reports because that would be very difficult. As soon as HIV is in the blood it’s a problem. What we could do is treat the blood with transfusions that have been treated with plasma beforehand, to remove the virus from the blood. This might [be] possible as we’ve tried to treat blood over a long period of time without seeing any damage to the blood cells.
Plasma seems to be to some extent and at certain doses selective and kills viruses without harming any blood cells. Of course, more intensive research has to be done because we haven’t tested it with the HIV virus yet.
The problem however is that once a patient has the HIV virus, it’s very difficult to get rid of it – it would be more of a preventative measure. Some media reports have also said you could ‘cure’ the common cold because we treated the adenoviruses with plasma. Also, this is going to be very difficult.
EF: What effect could plasma have on pharmaceutical drug development?
JZ: It could have quite a big impact on drug development, not really at present but in the future. We’re hoping that plasma itself will be a drug one day. For the first time we can design plasma as we desire and for specific applications. There are also newer results, also from our group, which show that plasma is not only selective with eukaryotic and prokaryotic cells but also with cancer and healthy cells.
There are possibilities to design the plasma in a certain way but we have to learn more about what it actually does. We have to look at signalling pathways in the cells to see what kind of stress the treatment builds on the cells.
The other thing we’re hoping to do is in relation to plasma pre-treatment of drugs. It is known that plasma makes tiny, tiny holes in the membrane of cell walls. That opens up the opportunity to get drugs into a cell much faster when they’re pre-treated with plasma. Other things which, however, are quite far in the future, are to insert the drug in some sort of gaseous form into the plasma so the plasma directly transports the drug to the cells.
EF: When could we see plasma-based drugs on the market?
JZ: Not for many years. We’ve started doing some research but this is a huge and complex area. We first have to decide what kind of drug we’re going to use and so on.
At the moment we’re working with different cancer cells and chemotherapeutics. We’re trying combinations of plasma and chemotherapeutics to see if it’s better and if it works faster. We’re on this, but it will definitely take several years.




