Indian company Sun Pharmaceutical Industries has received the US Food and Drug Administration (FDA) approval of the Biologics License Application (BLA) for tildrakizumab to treat patients with moderate-to-severe plaque psoriasis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
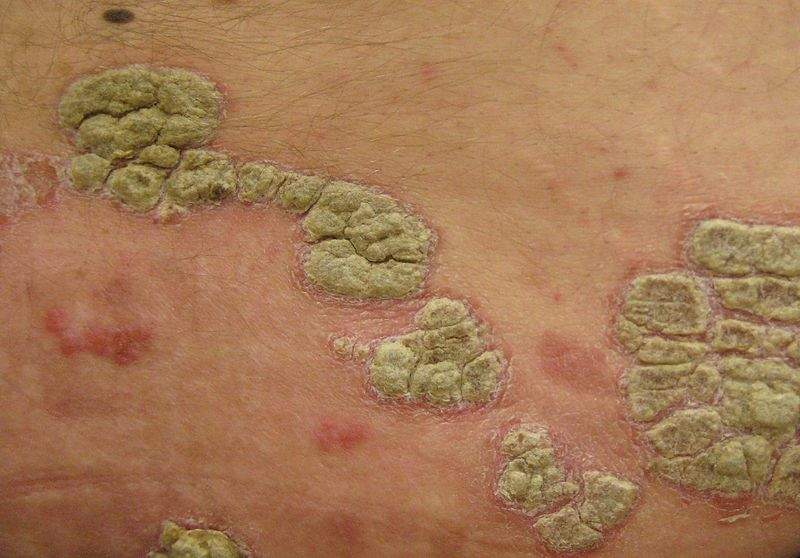
Psoriasis is a non-contagious disorder that appears on the skin and speeds the growth cycle of skin cells, resulting in thick scaly areas of skin.
The FDA filing acceptance follows acceptance of the regulatory filing of tildrakizumab by the European Medicines Agency (EMA) in March this year.
Tildrakizumab is a humanised, anti-IL-23p19 monoclonal antibody currently being evaluated and has been developed to selectively block the cytokine IL-23.
With the ability to target selectively, the treatment also has the potential to help control the pathogenic cells responsible for the inflammatory process of psoriasis with limited impact on the rest of the immune system.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSun Pharma North America Business chief executive officer Abhay Gandhi said: “At Sun Dermatology, we are committed to making a difference in the lives of patients and healthcare providers.
“The acceptance of the regulatory filing by the US FDA marks a significant milestone as we seek to advance for tildrakizumab as a potential new treatment option for people who continue to struggle everyday with the chronic nature of psoriasis.”
In 2014, worldwide rights of tildrakizumab were acquired by Sun Pharmaceutical Industries' wholly owned subsidiary from Merck.
The BLA filing for tildrakizumab was submitted to FDA by Merck and it was based on two randomised, placebo-controlled, multicentre, three-part Phase III trials (reSURFACE 1 and 2).
Both reSURFACE 1 and 2 were designed to demonstrate efficacy of tildrakizumab in moderate-to-severe plaque psoriasis compared to placebo and comparative drugs, as well as to assess safety and tolerability.
The trials involved more than 1,800 patients across 200 clinical trial sites, including some patients who have been treated with tildrakizumab for up to three and a half years.
Image: Plaques of psoriasis. Photo: courtesy of James Heilman, MD / Wikipedia.




