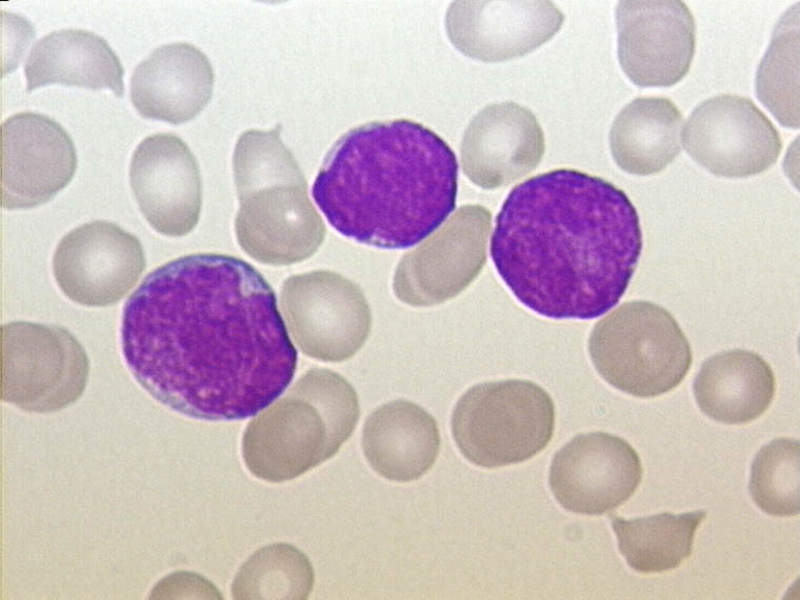

The US Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) has unanimously recommended approval of Novartis’ CTL019 (tisagenlecleucel) for the treatment of relapsed or refractory (r/r) B-cell acute lymphoblastic leukaemia (ALL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
CTL019 is an investigational chimeric antigen receptor T-cell (CAR-T) therapy that can be used to treat paediatric and young adult patients with r/r B-cell ALL.
CAR-T is different from other small molecule or biologic therapies as it is developed for each individual patient using their own cells.
While carrying out the treatment process, T-cells are drawn from a patient's blood and reprogrammed in the manufacturing facility to develop T-cells that are genetically coded to express a chimeric antigen receptor to recognise and combat cancer cells and other B-cells expressing a specific antigen.
Novartis Oncology CEO Bruno Strigini said: “The panel's unanimous recommendation in favour of CTL019 moves us closer to potentially delivering the first-ever commercially approved CAR-T cell therapy to patients in need.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We're very proud to be expanding new frontiers in cancer treatment by advancing immunocellular therapy for children and young adults with r/r B-cell ALL and other critically ill patients who have limited options. We look forward to working with the FDA as they complete their review.”
In paediatric and young adult patients with B-cell ALL that have relapsed multiple times or become refractory to treatment, the five-year period of disease-free survival is less than 10% to 30%.
The ODAC recommendation is based on the results from the Novartis-led first pediatric global CAR-T cell therapy registration trial ELIANA.
The trial involved patients enrolled from across 25 centres in the US, Canada, European Union, Australia and Japan.
Earlier this year, the company submitted a Biologics License Application (BLA) for CTL019 to the FDA.
Image: Acute lymphoblastic leukaemia (ALL), peripheral blood of a child. Photo: courtesy of Christaras A / Wikipedia.




