
Israeli-based Teva Pharmaceutical Industries has launched its generic of Novartis’ eye drop medication Pataday (olopatadine hydrochloride ophthalmic solution) 0.2% in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
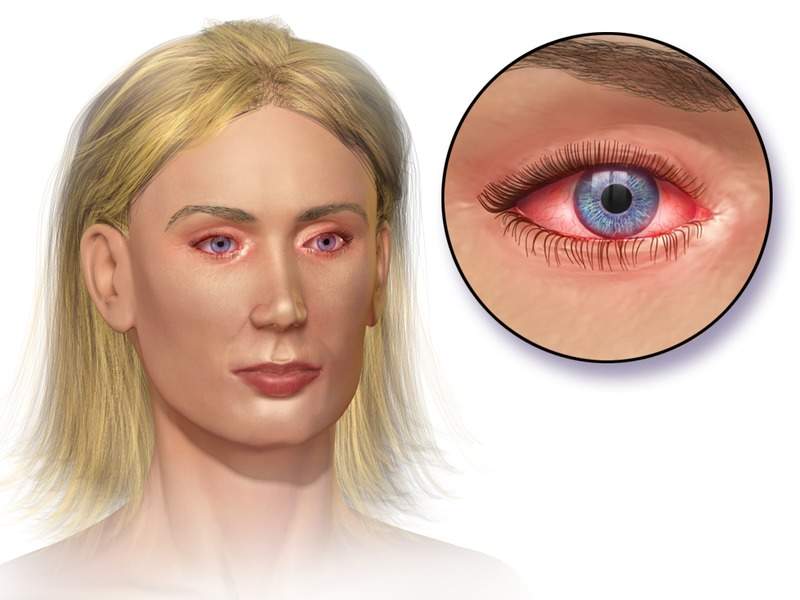
Olopatadine hydrochloride ophthalmic solution 0.2% is a mast cell stabiliser used to treat people suffering from ocular itching associated with allergic conjunctivitis.
Teva Pharmaceutical Industries global generic medicines president and chief executive officer Dipankar Bhattacharjee said: “Olopatadine hydrochloride ophthalmic solution 0.2% is an important treatment for our patients and a key addition to our generics product portfolio.
“This launch marks another successful first-to-file product for Teva, bringing the only generic version of this product on the market.”
Based on data collected by IMS, Pataday had annual sales of approximately $303m in the US.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOlopatadine hydrochloride ophthalmic solution 0.2% is developed for topical occular use only and should not be used in injections or administered orally. The therapy cannot be used to treat contact lens related irritation.
With approximately 600 generic medicines available, Teva Pharmaceutical Industries has the largest portfolio of US Food and Drug Administration (FDA)-approved generic products available in the market.
One in every six generic prescriptions provided in the country is currently filled with a Teva product.
In November last year, the pharmaceutical company received approval for generic Tribenzor (olmesartan medoxomil, amlodipine and hydrochlorothiazide) tablets in the US.
Olmesartan medoxomil, amlodipine and hydrochlorothiazide tablets are a combination of an angiotensin II receptor blocker, a dihydropyridine calcium channel blocker and a thiazide diuretic.
Indicated for the treatment of hypertension, the therapy is used to lower patient blood pressure.
Image: Illustration of allergic conjunctivitis. Photo: courtesy of BruceBlaus.




