Centocor, a subsidiary of Johnson & Johnson, announced in July 2004 the investment of €500m in the construction of a new state-of-the-art biopharmaceutical manufacturing facility in Barnahely, Ringaskiddy, Republic of Ireland. The design was completed by November 2004 and ground breaking was in July 2005.
Centocor merged with Ortho Biotech in 2008, and the new company renamed Centocor Ortho Biotech.
The design of the facility was completed by November 2004; groundbreaking followed in July 2005. Construction of the manufacturing plant was completed in mid-September 2007.
The facility is one of the safest large scale projects ever constructed in Ireland. In 2009, the facility was awarded the Facility of the Year Award for Sustainability.
It was constructed in Ireland to take advantage of generous tax advantages and incentive grants offered by the Irish Development Agency (IDA) and the Irish government, subject to European Union (EU) approval. Centocor also chose Ireland because of its location near to the company’s production base in Leiden in Holland.
Project
The facility was constructed on an IDA-owned, 100-acre brownfield site in Barnahely. The plant consists of a 10,000m² production building along with a central utilities building, a laboratory and administration block, waste water treatment plant and warehousing/storage. All of the buildings are linked along a central spine-link corridor. The four main buildings comprise a total floor space of approximately 24,391m².
The facility forms the strategic manufacturing centre of a global supply chain for many of the company’s existing and new pipeline recombinant mammalian cell fermentation and monoclonal antibody products.
The manufacturing unit at Barnahely produces products for the detection and treatment of many human diseases on a commercial scale but also is used to produce smaller quantities of drug material for clinical trials.
The plant employs more than 230 personnel, many of whom are pharmacists, chemists, process engineers and biopharmaceutical specialists.
Centocor contractors and construction
McCarthy Lynch designed the facility. The engineer, procurement and supply (EPS) contractor and construction management consultant for the project was SISK Pharma Division. The building was constructed using an advanced MUSKITA Mu-8XX curtain wall system integrated with Kingspan cladding which gives the advantage of superior thermal breaks to minimise cooling and heating costs throughout the year, as the maintenance of a constant controlled internal environment in a biopharmaceutical facility is of the utmost importance. Classic Building Solutions, of County Cork, carried out the external building work.
The facility includes bioreactors of commercial scale and clinical scale along with the associated class 100,000 cleanroom, media preparation, refolding and downstream processing and separation facilities (filtration and chromatography). The new plant also incorporates a process development section, where fermentation processes can be conceived and optimised to give the best possible yields of product, in addition to state-of-the-art plant and process automation to make it one of the most modern in Europe.
Low temperature refrigerated warehousing facilities are available for both products and ingredients as well as a small-scale fill and finish facility for packaging clinical quantities of products. Fill and finish is mainly carried out in Holland.
Centocor Remicade
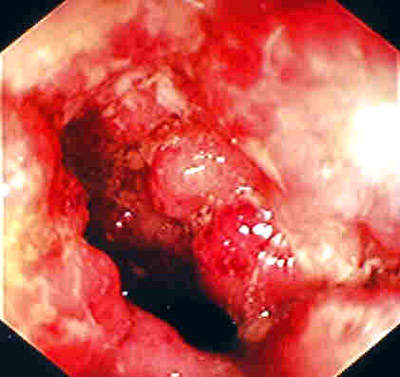
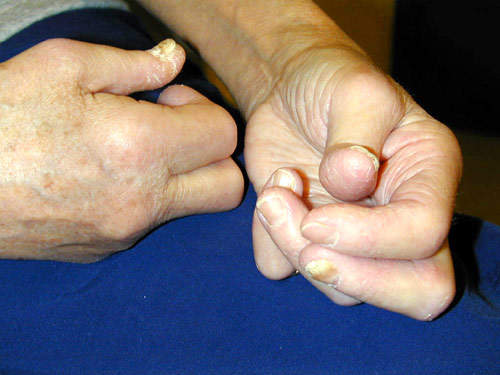
Remicade is a monoclonal antibody product is produced at the facility. The drug is used to treat autoimmune diseases such as rheumatoid arthritis (RA), Crohn’s disease and psoriasis that are caused by over-production of tumour necrosis factor α (TNF-α). The drug is highly successful, achieving sales in excess of $1.3bn in 2003 (being the first biotechnology blockbuster to do this).
Remicade was first approved in 1998 for RA and Crohn’s disease (it is the only FDA approved product for Crohn’s disease).
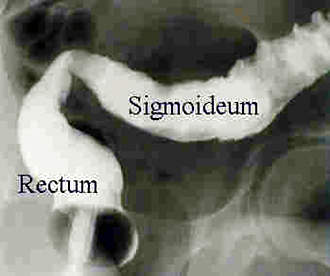
The company extended the usefulness of the drug by continuing clinical trials into other autoimmune diseases such as ulcerative colitis, psoriasis, ankylosing spondylitis, lupus erythrematosus and psoriatic arthritis.
The success of this drug, and others that were in the company pipeline such as Reopro (a heart medication), highlighted the need for the company to obtain extra production capacity, particularly at a time when rival drugs such as Enbrel and Humira are about to come to market.