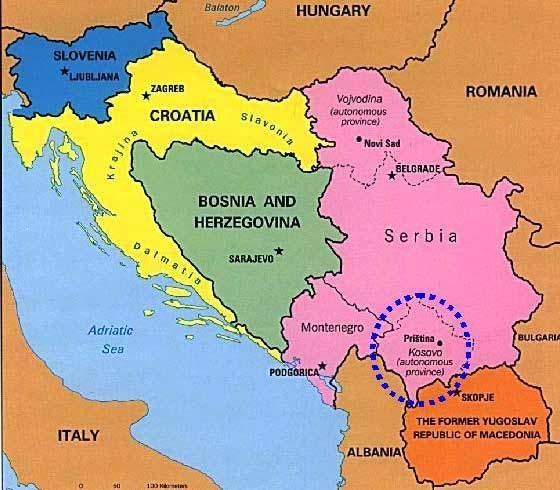
When the war in Kosovo ended in June 1999 the country, including its capital Pristina, was in a terrible state. Out of a total population of 2.2 million people, nearly 900,000 were displaced from their homes and then moved back. One of the main problems of moving refugees back to their homes was the reconstruction of a good health service and a regular supply of medicines, not just antibiotics but simpler lotions and shampoos for the treatment of conditions rife in refugee camps such as lice and scabies.
Since 2000, Kosovo started to develop a pharmaceutical regulatory and policy framework from literally nothing, but with the eventual objective of achieving European standards.
The agency established for the regulatory control of medicinal drugs in the province is the Kosovo Medicines Agency (KMA), which was established in 2001 through EC funding.
Its responsibilities included overseeing efficacy, efficiency and safety of the pharmaceutical products within Kosovo. The KMA has achieved its objective of meeting EU standards of pharmaceutical regulation by the end of 2006.
Nexhet Kondirolli pharmaceuticals
Nexhet Kondirolli has stepped into the breach, and with the help of European, USAID and venture capital funding, constructed a new pharmaceutical facility to supply much-needed medicines to Kosovo.
Nexhet Kondirolli first opened a small pharmaceutical production plant in 1992 that employed 23 staff. Unfortunately the Kosovan war destroyed the plant and any undamaged equipment was looted. In 2000 Kondirolli again rebuilt his pharmaceutical enterprise in a refurbished brick factory with a loan of $9,000 from the Microenterprise bank in Pristina.
Kondirolli set out to build a number of small production lines for the compounding and bottling of cough syrup and a range of 40 basic products from anticolic elixirs, ointments and creams to shampoo and basic pharmaceuticals in tablet form.
The technology was very primitive, basically machines were built by hand from cast-off bits and pieces of junk; at this stage the facility employed six people. During 2002–04 the company was making €0.5m per year and production was back to a third of pre-war levels.
New facility
The planning for a brand new purpose-built facility began in 2003. Niels Schwarz, a former director of operations for Merck, volunteered to help Kondirolli in developing a business plan for the new facility and to obtain permits, finance and eventually European Regulatory standards.
The UN Health Minister granted an operations permit in 2003 and with the help of USAID, funding was obtained from an Austrian venture capital company, Horizonte Venture Management, in January 2004. Additional funding for the employment of ten additional staff was also supplied by the United Nations Development Program (UNDP) in December 2005. Kondirolli has expanded his intended market to include ten million customers in Kosovo, Albania, Macedonia and Bosnia-Herzegovina.
Construction and the future
The construction of the new plant began in mid-2004 on a site near Pristina. The construction contract was granted to Siborra Panelli, a Kosovan construction company. The contract for outfitting the plant was awarded to suppliers in Germany and Turkey.
The 32,000ft² plant was structurally complete by the end of 2005 and underwent outfitting in early 2006 before undergoing the stringent commissioning and qualification process for achieving Good Manufacturing Practice (GMP) and European quality standards.
The concrete floor of the facility was laser-levelled to ensure airtight operations in the sterile ‘cleanrooms’ where medical infusion bags are to be produced. This was followed by construction of the building shell and then the interior labs, airlocks and cleanrooms required for GMP compliance.
Throughout the construction process USAID has continued to provide technical support to the fledgling pharmaceutical company and the construction company. When finally outfitted and complete the plant will have four production lines and two shifts, with 75 employees and an annual output of five million units.