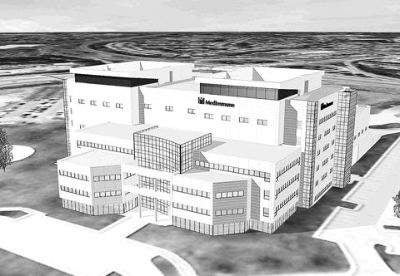
In September 2006 MedImmune, a Maryland-based biologics company working in the areas of infectious diseases, cancer and inflammatory diseases, started the construction of a new phase of its biologics manufacturing facility at the company’s Frederick site in Maryland, US.
MedImmune has been established in Maryland for 18 years and is best known for its production of FluMist, a nasal-spray flu vaccine (manufactured in Philadelphia). In April 2007, AstraZeneca purchased MedImmune for $15bn. The acquisition was to boost AstraZeneca’s presence in the biotech medicines sector and fitted well with Cambridge Antibody Trust, which AstraZeneca bought in August 2006 in a £580m deal.
It has since been incorporated into the former Cambridge Antibody Trust and other biological research being conducted elsewhere within AstraZeneca. MedImmune will help AstraZeneca’s plans to deliver an average of one new biologic product per year from 2013.
The first phase of expansion was completed by the end of 2009, and netted MedImmune a 2011 Facility of the Year Award in the project execution category.
Frederick expansion
The $250m expansion was the first phase of a multi-phase construction project first announced in October 2005. The facility has a manufacturing floor space of 710,000ft² (first phase 331,000ft²). More than 840 extra employees may potentially be added to the Gaithersburg HQ and R&D site, where further expansion is underway. The Frederick manufacturing facility has around 300 employees.
The expansion increased the company’s cell culture manufacturing production capacity, in preparation for the production of monoclonal antibodies.
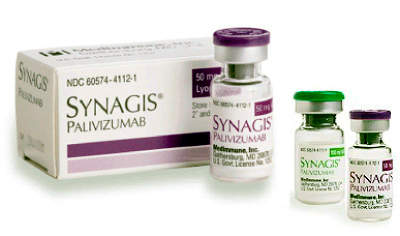
The facility has been built adjacent to a 150,000ft² site in Frederick that was built in the mid-1990s and is used to produce Synagis, a treatment that prevents some respiratory infections in babies.
The company already owned an additional 35 acres of land surrounding the Frederick facility and part of this was used for the expansion. The plant includes two additional commercial-scale bioreactors to produce monoclonal antibodies.
Contracts
In the third quarter of 2006, MedImmune was given a $170m, five-year contract from the US Department of Health and Human Services (HHS) to develop cell culture-based seasonal and pandemic vaccines. This facility is the production site for the vaccines. The contract is part of the US Government’s goal to provide enough capacity to protect every American citizen on a year-round basis.
In June 2009, the HHS awarded a $90m contract to MedImmune for manufacturing a monovalent live attenuated influenza vaccine for Influenza A(H1N1).
Construction
MedImmune designated Parsons as the architectural firm responsible for the design of the facility. MedImmune, the City of Frederick Department of Economic Development, and the Frederick County Office of Economic Development collaborated on the infrastructure necessary to support the site expansion.
The State of Maryland transportation department agtreed to spend more than $100m on road improvements in the area. The City of Frederick and the State of Maryland fought to keep the company from building in another state. The financial incentives put together to keep MedImmune were worth an estimated $19.5m.
The company’s plan is to turn the Frederick site into its primary manufacturing facility, while the Gaithersburg building will continue to house its research and development operations and corporate offices. There are already 1,000 employees in Maryland between the two sites (225 workers in Frederick and 775 workers in Gaithersburg).
New compounds
MedImmune already produces Synagis (palivizumab), the first monoclonal antibody approved by the FDA to help prevent an infectious disease, at the Frederick site. The company has about 30 drug candidates in development at the current time including potential treatments for lupus and some types of cancer.
Original plant
The Fredrick site was developed in 1996. The manufacturing facility required an investment of $30m to construct and the state and city provided a $13m incentive package.
The company originally purchased a 26 acre area of land in the Center Park business park, which included 15 acres for the 90,500ft² plant (expanded in 2001 to the current 150,000ft²) and 11 acres that the company intended to use for a new corporate headquarters, should it decide to move from Gaithersburg (ultimately the HQ was not moved and additional land was purchased for expansion).
The plant was completed by 1998 and in full production in 1999, producing an infant pneumonia drug called RespiGam. The general contractor was Riparius Construction; the construction manager was Fluor Daniels, and the architect/designer was also Fluor Daniels. Tri-State Drywall was also involved in the construction. The project consisted of experimental laboratories, Class 10M–100M cleanroom spaces, bio-secure manufacturing facilities and support office space.
Further builds
In October 2008, MedImmune opened a new biologics research and development facility in England to create more lab space for its growing number of staff. It will also allow the company to boost the number of candidate drugs that it can develop annually.
The 92,000ft² facility is named the Aaron Klug Building after the 1982 Nobel Prize Winner for chemistry and is a substantial addition to its existing Granta Park site in Cambridge.
This development followed the expansion of another of MedImmune’s major production sites in Nijmegen in the Netherlands, which can produce up to ten discrete products each year, equivalent to 6.5 million vials.