Novartis began production at its new vaccine manufacturing facility in Marburg, Germany, in April 2011. The plant produces rabies and tick-borne encephalitis (TBE) vaccines and associated supplies such as media, washing operation, buffers and adjuvants for national and international markets.
It has the capacity to produce 20 million doses a year of the rabies vaccine or 40 million doses a year of the TBE vaccine or a combination of both.
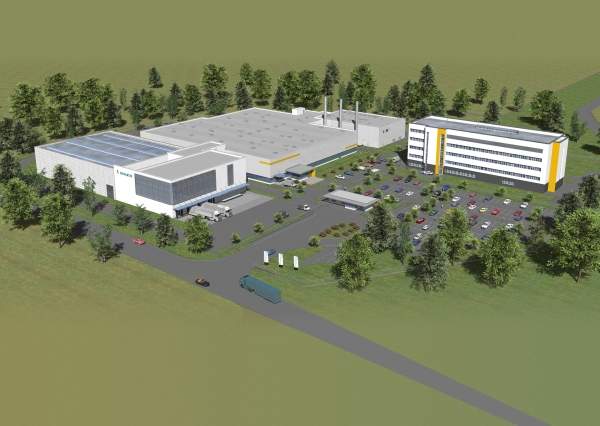
The plant is part of the company’s integrated facility known as the MARburg Site development (MARS) which broke ground in June 2008. The MARS is designed to serve as a Centre of Excellence for modern vaccine production.
Novartis invested about €145m ($198.4m) in the new facility, which represented one of its single largest investments in Germany. The foundation stone for the vaccine production building was laid in February 2009 with construction completed in a record time of 26 months.
The plant commenced production in April 2011. It is believed to have added 300 jobs (including 60 new positions) to the already existing workforce of 1,200 employees in Marburg.
The facility has won the Equipment Innovation award for its laser egg opening (LEO) machine in the 2011 Facility of the Year competition organised by ISPE, INTERPHEX and the Pharmaceutical Processing Magazine.
Novartis vaccine manufacturing facility specifications
Construction of the vaccine production unit at the existing MARS site was necessitated by factors such as on-site know-how, availability of infrastructure and the growing demand for rabies and TBE vaccines.
The original plant design was revised by the FDA, which facilitated installation of two different production lines in the same area. The lines can be used interchangeably for production of the rabies or TBE vaccines or simultaneously manufacture both.
This changeover system allows Novartis to produce vaccines as per the market demand and also reduce the cost of running dedicated lines.
Manufacturing of rabies and TBE vaccines
The vaccine production plant has a floor area of 2,700m2. It adopts lean manufacturing techniques based on process and equipment improvement.
The plant manufactures rabies and TBE vaccines in eggs through a state-of-the-art laser egg opening (LEO) machine. The use of automatic egg opener has replaced the manual process of opening eggs which used to increase the risk of cross-contamination due to contact and egg damage.
The LEO machine eliminates cross contamination and increases productivity by ten times when compared to manual operation.
The system automatically opens four eggs simultaneously by using four independent laser beams and has the potential to open 3,000 eggs an hour while maintaining aseptic conditions.
The production of rabies and TBE vaccines involves three steps which include cell suspension, virus propagation and concentration of the inactivated virus. Egg opening is a part of the cell suspension process.
A conveyor system equipped with optical sensors transports the eggs and places them in to the trays inside the machine. The sensors detect the exact position of the tray and the egg, enabling the laser to fall exactly on the eggs present in the tray.
Contractors involved in the facility development
Turner & Townsend were the main contractors for the project. They were involved in the engineering, execution and start-up of the facility.
MARS project
The integrated site occupies a total area of 8.4ha. It houses a 3,000m2 raw material storage facility, media, buffer production solutions and vaccine boosters area with 3,800m2 of floor area, cleaning operations, monitoring and control room and a power house.
The 115m long passage in the ground floor connects to all the buildings.
The MARS site also houses a new quality control building in addition to the production building. The quality control building was opened in February 2010 after 15 months of breaking ground.
It occupies an area of 8,000m2 and serves as a quality control centre of all of the Marburg facilities. It conducts 35,000 to 40,000 analytical tests and monitors more than 100,000 samples annually.
The warehouse occupies an area of 3,000m2 and has 4,000 pallet spaces with a provision for chilled as well as room temperature storage facilities. A long corridor connects it to the production facility.