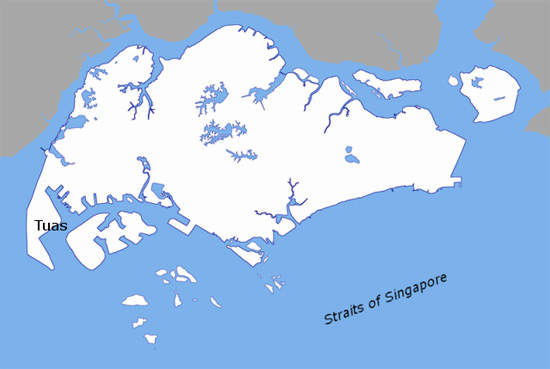
Novartis is a multinational pharmaceutical company, based in Basel in Switzerland. The company has plants situated across the world but is developing a particularly strong presence in Asia, with its Asia Pacific HQ in Singapore. One of the latest additions to the Novartis group of facilities is a tabletting plant opened in October 2007 in the Tuas Biomedical Park in Singapore.
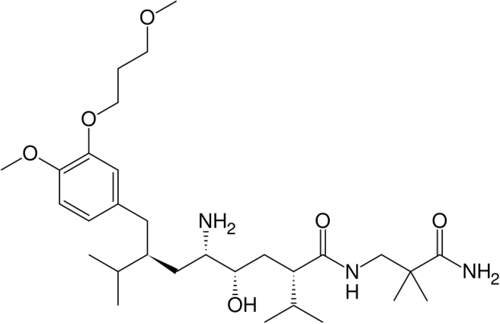
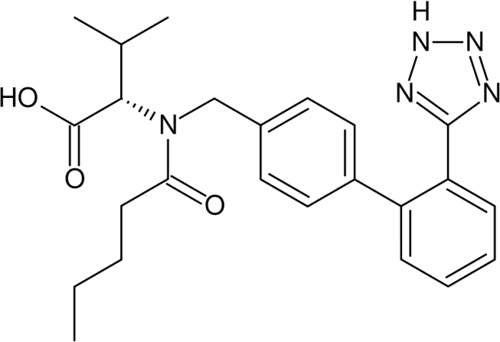
The new plant will produce a range of solid dosage forms for the Asian, US and Middle Eastern markets, including the well-known Novartis medications for hypertension, Diovan (valsartan) and Tekturna (aliskiren).
The new $180m facility will initially employ around 160 personnel and will have the capacity to manufacture about 3.3 billion tablets a year.
At the same time as the opening of the Tuas plant, Novartis took the opportunity to announce the construction of one of its largest biopharma manufacturing facilities ever in Singapore. This site was chosen from 40 potential sites worldwide.
This has made it clear that Novartis is actively developing its biologics manufacturing capacity, with 25% of the company’s research programme involving biologics.
The company is however cost cutting in other areas – losing 1,260 jobs worldwide to save $230m. This new plant also demonstrates that the company is increasing its presence in Asia.
NEW BIOLOGICS FACILITY
The new Novartis biotech manufacturing facility is expected to require an investment of around $700m and will start construction in the second quarter of 2008. The new plant will represent one of the largest plant investments Novartis has ever made and will contain the company’s seventh and biggest large bioreactor, with a capacity of around 40,000l.
The new facility will employ 300 personnel and is expected to be opened, validated and certified by 2012. The facility is being designed to manufacture both clinical and commercial quantities of new biopharmaceuticals. Most of the therapeutics will be monoclonal antibodies produced using state-of-the-art techniques and will be used to treat conditions such as rheumatoid arthritis, oncology and asthma.
Some of the latest Novartis biologics include Tasigna, a second-line therapy for leukaemia patients with Philadelphia chromosome-positive (Ph+) CML who cannot tolerate conventional treatment, and also the blockbuster cancer drug Gleevec.
CONTRACTS
In January 2008 Novartis appointed Turner and Townsend as one of the contractors for the construction of the new facility. Turner and Townsend will be involved in tender preparation and negotiation and evaluation of the construction manager, with the commission potentially extended.