Roche operates in China as Shanghai Roche Pharmaceutical Co Limited, which is a highly successful joint venture operated in conjunction with a Chinese partner Sunve Pharmaceuticals of Shanghai, China, and was started in 1994.
Sunve, which is a highly successful Chinese pharmaceutical company, has two joint ventures with Roche and also Givaudan for pharmaceutical preparations and flavour and fragrance production respectively that is Shanghai Roche Pharmaceutical Co Ltd and Shanghai Givaudan Ltd.
Sunve’s four main areas are cardio, cerebro-vascular, immune system and oncology.
SHANGHAI HIGH POTENT PRODUCTION PROJECT
The Shanghai High Potent Production Project (SHiP) was based on a small budget of only $16.64m and has consisted of the construction of a new specialised manufacturing building for the production of highly active medicines requiring strict containment. The products are manufactured in the tablet and capsule form and are for distribution in the domestic Chinese marketplace, and also for international export.
The products will include substances for the prevention of organ rejection in transplant recipients and for the treatment of specific forms of cancer. The whole focus of the SHiP project from conception to completion was about speed, innovation, technology and a successful global team effort including European and Chinese engineers and technicians.
The resulting new facility was constructed in only two years starting November 2004 and following validation has been in commercial production since mid-2006. The facility includes state-of-the-art technology for environmental safety, protection and containment.
PROJECT
Detailed design of the 2,100m² facility was completed in November 2004 and construction commenced in April 2005 following financial and planning clearance.
The construction was completed by October 2005 and fitting out was completed shortly after this. From the last quarter of 2005 until mid 2006, the plant underwent validation for cGMP and by the FDA and EMEA as well as Chinese regulatory authorities. In April 2006 commercial production began.
Project management and start-up were administered by Shanghai Roche Pharmaceutical and the architects and construction managers were Meissnert and Wurst Zander (Shanghai) Ltd along with several Chinese partners.
PRODUCTS MANUFACTURED
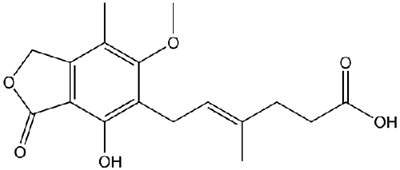
The plant now manufactures products such as Cellcept (Mycophenolate Mofetil), Xeloda (Capecitabine), and Furtulon (Doxifluridine) for both the local and export market.
Cellcept is an immunosuppressant acting on blood cells. Xeloda is a chemotherapy drug that is given as a treatment for some types of cancer, including advanced bowel cancer or breast cancer. Furtulon is an orally administered anticancer drug prescribed against malignant tumours.
FACILITIES
Pharmaceutical companies have developed better and more potent active ingredients, as cytotoxics for oncologic use but concurrently there has been increased attention to the problems of health and safety, industrial hygiene and the environment.
Because of a combination of these it is now the policy to produce such drugs in super-high containment facilities over and above the facilities in the normal production environment.
The plant design in Shanghai has incorporated the latest technology in containment for high potency powders.
In particular there are permanent physical barriers between products and operator, negative pressure isolator containment to ensure safety in the event of glove malfunction or accidental door opening and isolator chamber cleaning systems as well as cleanroom facilities, airlocks and facility for spillage containment contingency.
There are various types of isolators, from a complex double chamber system to special connection systems such as external boxes for material ingress/egress.
The use of isolators is consistent throughout the entire production plant from dispensing operations, through reactor loading and centrifuge unloading, to final product unloading from the drier.