In the second quarter of 2003 the Swedish vaccine producer, SBL Vaccin AB, brought on-line a major extension and upgrade to the vaccine production facility at Solna near Stockholm. The company has been in various hands and used to be a subsidiary of Powderject PLC, which acquired the company in a $50m deal in 2001. Powderject PLC itself merged with the US vaccine manufacturer Chiron Inc in May 2003.
The SBL Vaccin name was retained and products were marketed under the brand name in Europe and Asia. Chiron however sold the company in 2004 because it was not aligned to their core business. The company went into private hands in a management buyout supported by venture capital from 3i and SEB Företagsinvest. In 2006 the company was sold again to the Dutch biotech company Crucell in a deal worth €39.4m.
The extension to the Solna plant was started in the first quarter of 2002 and required an investment of $7.5m from the parent company Powderject PLC. The new extension increased the production of cholera vaccine at the facility.
The cholera vaccine produced in Solna is the only one that has full World Health Organisation (WHO) recommendation. The vaccine was then incorporated into a new combination orally administered vaccine product aimed at the travel market called Dukoral.
CHOLERA VACCINE FACILITY CONTRACTORS
The contract for basic engineering and design of the plant, including a biosafety level three production area, was awarded to Elomatic Pharmaceutical Engineering of Finland. The contract for the design, engineering, control and automation of the bioreactors and associated downstream processing was awarded to Belach Bioteknik AB of Sweden.
The control and automation system software and IT was provided by Wonderware Scandinavia. Software systems to integrate the production plant with the sales and distribution side of the business were provided by Jeeves (Sweden) in collaboration with ISE Konsult.
VACCINE PRODUCTION PLANT
The vaccine production takes place in three different buildings at the plant. The first is the bulk solution preparation building, where media preparation and bulk solution preparation for diafiltration and cleaning are carried out. The second building houses the wastewater inactivation system, where all process solutions are collected, monitored, pH adjusted and inactivated. The third building houses the four bioreactors used for bacterial stem cultivation and also inactivation process vessels and ultrafiltration equipment.
VACCINE PRODUCTION
The vaccine production process begins with frozen cholera stems; these are thawed and inoculated to the bioreactors containing bulk media. Fermentation is followed by downstream processing. This includes ultrafiltration to remove debris and aggregated material and bacterial inactivation or attenuation by using heat or treatment with a formaldehyde solution.
Several washing and filtration steps are followed by transfer to a sterile container and cold room storage. The vaccine is then passed to a fill and finish line to be dispensed and packaged.
TECHNOLOGY
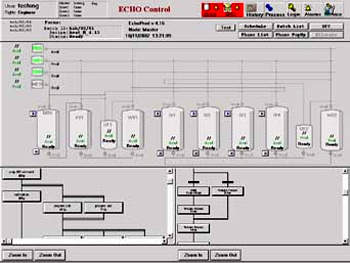
Since the operation cultivates a bacterium which is highly hazardous to human health the plant is highly automated, making use of Wonderware’s InControl, InBatch, InTouch and FactorySuite software systems. The InBatch system is designed to run parallel batches in four inactivation vessels to collate all of the batch related information, including operator information, material tracking data and process parameters, and to control each batch process.
The batch control and automation system satisfies FDA 21 CFR Part 11 and also Good Automated Manufacturing Practice (GAMP) guidelines. The bulk solution preparation areas and waste inactivation areas are largely software-controlled in continuous mode operation with little operator activity. The plants may be observed remotely in a control room area.
SOFTWARE
InControl is the software that provides basic and sequential control and also phase logic; this is the software that controls the equipment. InTouch is the software that provides the Human Machine Interface (HMI); this allows the operators to access process control parameters and process data in a user-friendly graphical format in real time.
InBatch is the batch management software, controlling not only batch processes but also logging all the pertinent data for each batch for a reliable, foolproof tracking system.
Belack Bioteknik specialise in the design, construction and installation of highly automated bioreactor systems for biotech applications. They have also made automation improvements to the polio production system at SBL Vaccin during 2003. The cholera production capacity is seven times greater than before the expansion.
DUKORAL COMBINATION VACCINE DRINK
The new expansion at Solna is to concentrate on the production of Dukoral, a combination vaccine drink, which is the company’s core product. The vaccine will protect against enterotoxigenic E. Coli (ETEC), a major cause of diarrhoea for travellers, and also cholera, a major risk in underdeveloped countries caused by contaminated food and water.
Dukoral is licensed and available in more than 50 countries worldwide including the US and is marketed in conjunction with Aventis Pasteur. Sales exceeded €14.7m in 2005, representing a dramatic increase of over 60% since 2004. Aside from Dukoral SBL Vaccines market and sell vaccines, produced by international pharmaceutical companies, in the Nordic market. The company’s total turnover in 2005 was €38m.
The oral dosage regimen of this product is very convenient and because of this it is currently being supplied to African and Latin American countries where cholera is a problem on the recommendation of the WHO. Dukoral has not yet been approved by the FDA for the US market.