Takeda Chemical Industries Ltd broke ground on the construction of a new active pharmaceutical ingredients (API) plant in Clondalkin, South Dublin County, Ireland, in early 2003. Construction of the 80,000m² plant started in October 2003 and was mechanically complete by early 2005. The plant was fully fitted out and validated by mid 2005 and in commercial production by late 2005. The new plant makes major contributions to pharmaceutical product supply for Western Europe, process research and cost competitiveness.
The initial investment for the project was €90m ($105m) and the new facility was built on a 20-acre site at Grange Castle International Business Park. The plant is the first API plant built by Takeda outside Japan and complements the pharmaceutical finishing plant located in Bray, County Wicklow, Ireland.
The API plant is run by Takeda Pharma Ireland Ltd (TPI), a subsidiary company set up in 2002, whereas the finishing plant is run by Takeda Ireland Ltd. Takeda has employed new personnel for the new plant. Approximately 60 staff were required for positions in QA, QC and process research and engineering.
In July 2009, Takeda Pharmaceutical integrated TIL and TPI into a single operation. The integration included a complete transfer of TPI’s assets to TIL. TPI has been liquidated following the transfer and other required formalities.
Clondalkin plant lead contractors and designers
The design, management, procurement and construction contract for the new plant was awarded to Foster Wheeler Energy on a joint basis with Ishikawajima-Harima Heavy Industries Co Ltd (IHI). Project Management Group was also involved and formed part of the core design and construction management team assembled by Takeda. The 20 acre site for the project at Grange Castle was sold to Takeda by IDA Ireland.
PM’s responsibilities included the compilation and submission of the planning application, environmental impact statement and fire certificate applications. The IPC (Integrated Pollution Control) licence application was also PM’s responsibility.
Design responsibilities included site infrastructure and civil, structural, architectural and building services for all buildings with the exception of the main production building. Piping design was also included for areas outside of the main production building.
The planning and design process started in 2002 with initial facility design, an environmental impact study and planning permission being sought from and granted by South Dublin County Council. The new facility was based on a modular construction principle. Since the timescale was so short this saved a great deal of time. Modular constituents of plant were constructed and pre-validated before final installation at the site.
In addition, the modular plant approach allows the phase one plant to be expanded more easily at a later date. Phase two expansions of the plant are already being considered; if the economic climate remains buoyant Takeda may well need the plant to have an even larger production capacity.
Batch control system at API plant
Yokogawa UK supplied an advanced batch control system for the new plant. The plant was specifically designed to be multipurpose, allowing a large number of products to be manufactured. This necessitated the use of a comprehensive batch control system, and Takeda decided to use Yokogawa’s CS3000 DCS Batch Control system, which is based on the ISA S88.01 Batch Standard.
The CS3000 Batch Control system also complies with the current FDA requirements for the use of electronic records and electronic signatures (as specified in FDA 21 CFR Part 11). The system comprises a Centum CS3000 R3 with eight operator stations, one audit trail server, five field control stations, 4500 conventional I/O points, and Profibus communications to the packaged equipment. In addition to the batch control functions, it produces online batch and process reports.
Sub-contractors for API plant systems
Mercury Engineering Ireland carried out mechanical services and installed fire protection technology on the plant. This contract included a mechanical process package and building services package across the entire complex. The total pipe work installed was in excess of 30,000m. The pipe work supplies water of various grades, gases and steam. It also takes away waste for treatment.
BMD Mechanical Engineering carried out the offsite fabrication of 19 pre-assembled pipe racks for the new plant and 10,000m of process and utility piping.
Rohcon Construction was responsible for fitting-out the plant. These works involved the building fit-out works for a five-storey production building, combined utility building, warehouse and administration–laboratory building. The contract included dry-lining works, doors and all specialist wall and floor finishes, cleanroom construction, fire stopping and sanitaryware. Rohcon also completed the internal lift shaft walls to facilitate the lift installation.
Rohcon’s work was heavily integrated with equipment installation and other contractors’ work; as a result work areas were only made available for limited periods.
Therefore, to achieve the overall project programme, all works involved close cooperation with the design team and fellow contractors. The quality of the building finishes installation was critical in that they needed to be of the highest standard to achieve the technical requirements of GMP.
Takeda products and predictions
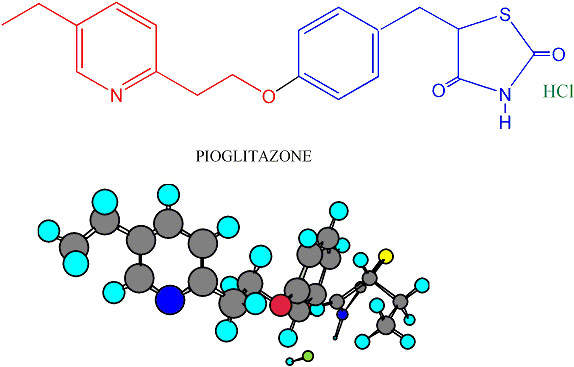
The API plant produces the active ingredients for its Type II diabetes drug pioglitazone hydrochloride (Actos). The drug is then processed at the finishing plant in Bray. In addition, the new API plant produces a range of drugs for use in clinical trials. The Takeda production network covers Japan, Asia, and in Europe, Italy and Ireland.
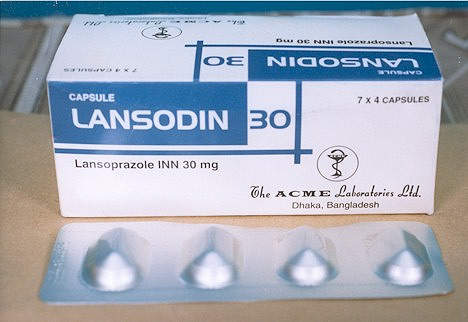
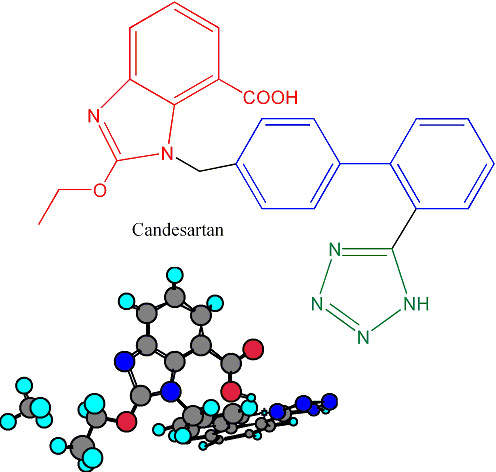
The operations in Ireland are responsible for the production of the hypertensive drug candesartan (marketed as Blopress, Amias or Kenzen), the peptic ulcer drug lansoprazole (Prevacid, Lansox, Lansodin) and other products for both the US and European markets. The API plant works in combination with the Hikari Plant in Yamaguchi Prefecture, Japan, to enhance the capacity of delivering bulk products on a global basis.