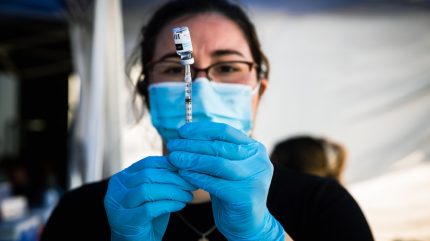
Following Bavarian Nordic’s announcement of the US commercial launch of its mpox (monkeypox) vaccine Jynneos (Imvanex/Imvamune), Morris & Dickson has shared that it will be the first US distributor of the vaccine for the rare viral disease.
According to the 3 March press release, the Caddo Parish, Louisiana-based wholesaler is receiving the first shipment of vaccines at an undisclosed date. Morris & Dickson has a storage and transport capability which is a “unique” attribute of the company’s distribution techniques. As per Jynneos’ label, the vaccine must be kept frozen at -25°C to -15°C and protected from light.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
“Jynneos meets a critical public health need and helps ensure equitable access to healthcare, which in turn helps significantly prevent the spread of mpox to at-risk populations,” said head of Specialty at Morris & Dickson, Layne Martin in the announcement.
The two-dose, subcutaneously administered vaccine regimen, which first gained US Food and Drug Administration (FDA) approval in September 2019, was recommended for routine usage in adults with specific mpox infection risk factors by the Advisory Committee on Immunization Practices of the US Centers for Disease Control and Prevention (CDC) in October 2023. Previously, the committee recommended the vaccine for this population only at the time of an outbreak.
Jynneos is based on a live non-replicating MVA-BN strain of the modified vaccina virus Ankara which is not able to replicate in human cells and induces a strong cellular and humoral immune response. The vaccine has been a heavy contributor towards Bavarian Nordic’s revenue surge as the company reported a preliminary revenue of $1bn for 2023.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData



