Ligand Pharmaceuticals’ wholly owned subsidiary LNHC has concluded its merger with Channel Therapeutics’ CHRO Merger Sub, resulting in the formation of a new entity, Pelthos Therapeutics (PTHS).
In conjunction with the merger, Pelthos secured $50.1m in equity capital. This includes contributions from investors led by Murchinson and Ligand itself, investing $32m and $18m respectively into Pelthos’ Series A convertible preferred stock and common stock.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Part of this investment involved the cancellation of $18.8m in bridge financing provided to support Zelsuvmi’s (berdazimer) commercial launch since early 2025.
Ligand will receive a 13% royalty on global net sales of Zelsuvmi.
Ligand CEO Todd Davis stated: “Today marks not just a corporate milestone, but a transformative turning point for the team at Pelthos.
“With the successful closing of this merger and the launch of Pelthos as an independent, publicly traded biopharmaceutical company, we have unlocked new potential for innovation and long-term value creation for our shareholders.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“This moment is the result of the vision, hard work and unwavering commitment of our internal team, who developed the commercial platform to help bring Zelsuvmi, a novel product addressing a significant unmet need, to market this summer.”
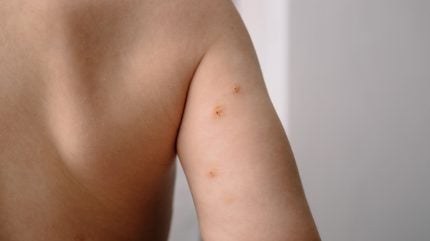
Pelthos’ immediate plans involve focusing on launching and commercialising Zelsuvmi topical gel at 10.3% concentration to treat molluscum contagiosum infections (a widespread poxvirus infection) in adults and in children aged one year and above.
With US Food and Drug Administration (FDA) recognition as a new drug, Zelsuvmi becomes a prescription medication approved for molluscum that can be administered by patients, parents and caregivers.
Pelthos continues to assess potential directions for Channel’s NaV 1.7 development programmes aimed at treating chronic pain, acute and chronic eye pain conditions, and post-surgical nerve blocks.
To facilitate this transaction process, Latham & Watkins was appointed as lead counsel to Ligand.




