The US Food and Drug Administration (FDA) has granted approval for Ascendis Pharma’s Skytrofa (lonapegsomatropin-tcgd, developed as TransCon hGH), a prodrug of somatropin or human growth hormone (HGH), to treat adults with growth hormone deficiency (GHD).
GHD is characterised by a decrease or complete lack of production of endogenous growth hormone.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Skytrofa was previously approved by the FDA in 2021 to treat children and paediatric patients aged one year or above weighing at least 11.5kg and are experiencing growth failure due to inadequate secretion of endogenous growth hormone (GH).
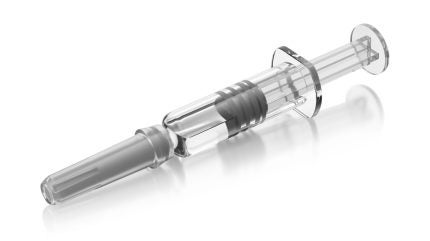
It is a sterile, preservative-free lyophilised powder that appears white to off-white, available in a single-dose, dual-chamber prefilled cartridge.
This therapy is administered subcutaneously once a week, ensuring a sustained release of active, unmodified somatropin.
The FDA’s approval for adults with GHD was based on the findings from the Phase III parallel-arm, randomised, active-controlled (open-label) and placebo-controlled (double-blind) foresiGHt trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt evaluated the safety and efficacy of weekly TransCon hGH in comparison to a weekly placebo and daily somatropin in adults diagnosed with GHD.
Ascendis Pharma president and CEO Jan Mikkelsen said: “Our market research shows Skytrofa is the treatment of choice for paediatric GHD among patients and physicians, and we are pleased to expand its availability in the US for the treatment of adults initiating therapy or switching from another growth hormone therapy.
“This important milestone is the first of many planned label expansions supporting our goal to become the leading endocrinology rare disease company.”
Last year, the company submitted a supplemental biologics licence application to the FDA for TransCon hGH to treat adults with GHD.




