On 14 August 2025, the US Food and Drugs Administration (FDA) approved Precigen’s Papzimeos (zopapogene imadenovec) for recurrent respiratory papillomatosis (RRP) – the first FDA-approved therapy for this rare airway disease. Patients with RRP experience the growth of benign tumours in the respiratory tract, forcing them into repeated surgeries to restore breathing and swallowing, with no pharmacological options available in the US or European Union up to now. Papzimeos’ approval therefore represents a turning point for patients and for regulators increasingly willing to accelerate therapies in rare diseases.
Papzimeos is a recombinant vector vaccine immunotherapy, utilising the adenoviral vector, stimulating both antibody production and T-cell activation against human papillomavirus (HPV) antigens. The drug is designed to generate an immune response directed against HPV 6 and HPV 11 proteins in patients with recurrent respiratory papillomatosis.
Full approval was based on results from the pivotal Phase I/II trial (NCT04724980) led by the National Institutes of Health (NIH), in which 51% of patients achieved a complete response with durable benefit at two years, providing the basis for regulatory review. The FDA’s decision to approve based on early-to-mid-stage clinical data illustrates a growing trend of flexible regulatory pathways where the unmet need is severe, and patient populations are small. This approach mirrors the agency’s broader strategy in gene and cell therapy, in which traditional randomised trials may be impractical.
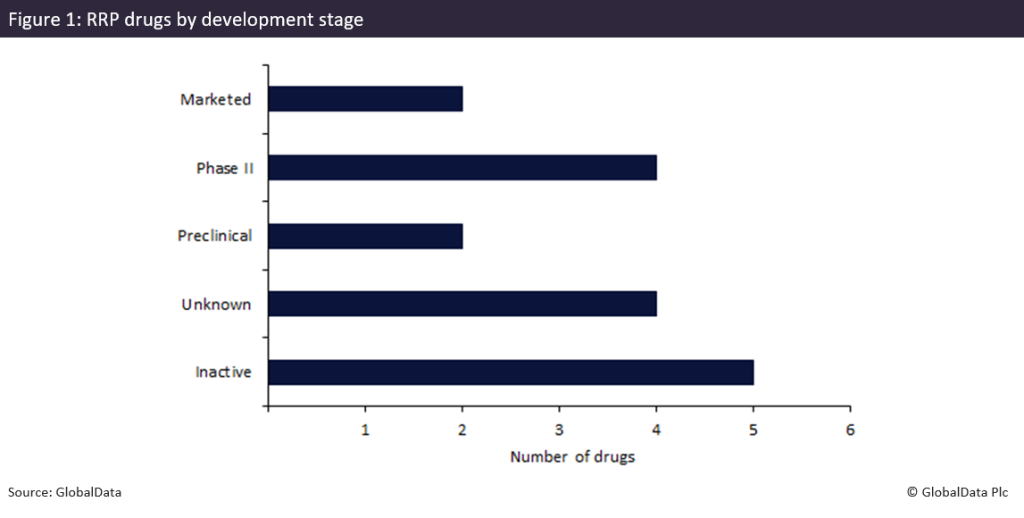
Figure 1 highlights the limited breadth of the RRP pipeline, with only two marketed therapies — Papzimeos as the first FDA-approved drug and interferon (a2b) by Pharmstandard, marketed in Russia. The latter has been locally approved for RRP and historically used as an adjuvant therapy, with limited adoption outside of Russia due to safety concerns, mixed long-term efficacy and a lack of broader international regulatory recognition. Beyond these two drugs, the pipeline is heavily weighted towards earlier stages: four drugs are in Phase II development, two candidates are in preclinical stages and four programmes remain of unknown status. Notably, a further five projects are currently inactive, reflecting the considerable attrition and recruitment challenges of this narrow patient population. The bottleneck at Phase II underscores how rare disease trials often stall before advancing into later stages of development, leaving the treatment landscape still remarkably thin.
Papzimeos’ arrival marks the second therapy available for RRP patients, expanding options beyond surgery and the limited use of interferon (a2b) marketed in Russia. Its approval sets an important precedent for future drug development in this space. However, with the European Medicines Agency yet to signal its stance, and with most pipeline assets still trapped at early stages, global access may remain uneven. Much like other rare conditions, the field now hinges on whether this first approval catalyses wider investment — or whether Papzimeos will remain a solitary breakthrough in an otherwise stagnant therapeutic area.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData