
This article explores the typical fill-finish process, the potential impact on unstable drug products, and best practices to protect these products during formulation, filling, storage, and distribution. It also examines common types of drug product sensitivities and outlines effective strategies to safeguard product integrity throughout the manufacturing lifecycle.
A typical fill-finish process
Before considering the modifications necessary to accommodate sensitive or unstable drug products, it’s important to understand the foundational steps of a typical aseptic fill-finish process. This process generally unfolds in three phases:
- The preparation phase
- The execution phase
- The review and release phase
1. Preparation phase
The project begins with a meeting between the client and the contract manufacturing organization (CMO). The client shares all critical product and process information, including:
- Tech transfer documentation
- Lyophilization (Lyo) cycles
- Formulation and pH ranges
- Analytical testing
- In-process and release testing requirements
This information enables method development and the creation of a Master Batch Record (MBR)—the primary guide used by all team members during the cGMP fill-finish run.
Following review and approval, an engineering run is conducted to test the process under non-GMP conditions. This run helps identify and resolve any issues before moving into full GMP production.
2. Execution phase
The execution phase begins with the formulation of the bulk drug product. Formulation typically takes place in a Grade C, Class 10,000 cleanroom. Depending on the product, this step can range from a straightforward thaw, mix, and filtration process to more complex procedures that involve reconstituting a powdered drug substance and adding various excipients. Each formulation process is distinct and may vary in the level of time, handling, and oversight required.
All formulation parameters—such as mixing duration, in-process analytical testing, and precise excipient quantities—are meticulously documented in the batch record and verified by a second team member for compliance and accuracy.
Following formulation, the bulk drug product is either:
- Held overnight or moved directly to filling
- Connected aseptically to the filling line
- Filled using peristaltic or piston pumps
Formulation and filling activities are generally conducted at room temperature and under LED or fluorescent lighting.
Once filled, the drug product is stored in a temperature-controlled work-in-progress unit, typically set at either 20–25°C or 2–8°C, depending on the product’s stability profile.
With the product now filled and stored under controlled conditions, the process moves into the final stage: review and release.
3. Review and release phase
During the review and release phase, the filled drug product undergoes a thorough quality check. This includes a 100% visual inspection followed by an Acceptable Quality Limit (AQL) inspection to ensure that the product meets specified quality standards.
If the product passes these inspections, it is moved to designated released product chambers. These chambers are maintained at the final storage temperature and are designed to hold the drug product while it awaits in-laboratory testing, microbiological results, and a comprehensive quality review of the batch record.
Once test results are confirmed, all deviations have been addressed, and Quality Assurance (QA) has completed its review of the batch record, QA initiates the official release of the drug product.
Following release, the warehouse team prepares the product for shipment. Depending on the client’s preferences, the product may be shipped directly to a clinical site, returned to the client, or transferred to another CDMO responsible for packaging, labeling, storage, and distribution.
These three phases: preparation, execution, and review & release, are what a typical fill-finish project from start to finish looks like. Any additional batches are much less involved since the protocols, test methods, and material specifications have all been created and approved.
Modifying the fill-finish process for unstable drug products
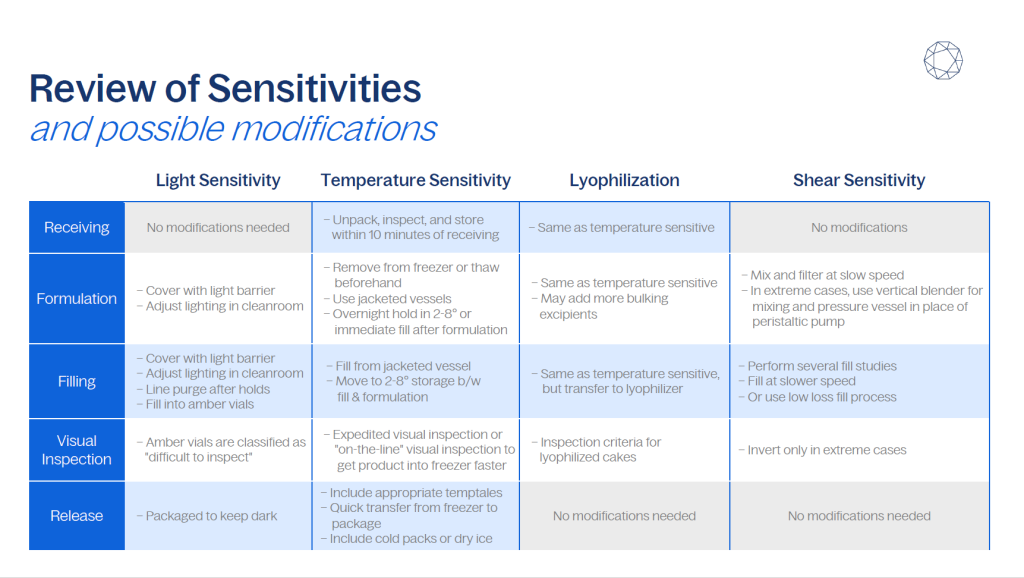
For drug products with specific sensitivities, such as light, temperature, or shear, following a standard fill-finish process may compromise product integrity.
For instance, light-sensitive compounds can be adversely affected by exposure to the LED and fluorescent lighting commonly used in formulation suites, filling rooms, and cold storage areas. Similarly, products sensitive to temperature may not tolerate the ambient conditions under which formulation and filling are typically performed. Additionally, shear-sensitive products can be degraded by the mechanical stress introduced during mixing and pumping.
Fortunately, the fill-finish process can be adapted to accommodate these sensitivities. With proper planning and execution, modifications can be made to protect drug products during formulation, filling, visual inspection, and storage.
The following sections explore three common types of drug product sensitivities and describe best practices for adjusting the fill-finish process to preserve product quality.
Protecting light-sensitive drug products
Light-sensitive drug products are typically vulnerable to degradation when exposed to higher-energy wavelengths of light—particularly those in the blue, purple, and ultraviolet ranges. In some cases, products may be affected by other portions of the light spectrum as well.
This sensitivity poses a challenge in environments where fluorescent or white LED lighting is standard, as these sources emit broadly across the visible spectrum and can damage the product. Fortunately, CDMOs can overcome this by keeping the product in the dark and, if the sensitivity is extreme, by removing the unwanted wavelengths in the lights used.
How to handle formulation for light sensitive products
To protect light-sensitive drug products during formulation, CDMOs can shield the drug solution by wrapping the mixing vessel, tubing, filters, and sterile containers to minimize exposure to light. For products with heightened sensitivity, amber-colored containers and light-blocking tubing may be used, or the formulation can be performed in a controlled environment specifically designed to limit light exposure.
In more extreme cases, the lighting in the cleanroom itself can be modified. This may involve covering windows and placing sheeting or tubing over light fixtures to filter out harmful wavelengths. Lighting systems can also be adjusted to emit only specific wavelengths, like green light, to ensure product stability throughout the formulation process.
How to handle filling of light-sensitive products
Filling presents its own set of challenges for light-sensitive products. To maintain protection, the solution and tubing can remain wrapped as they move through the filling setup. When using an isolator for sterility, fill lines can be covered up to the point of entry into the isolator.
Inside the isolator, the product may be exposed to ambient light. Although exposure time is typically brief, additional precautions—such as flushing the fill line and including an in-process or overnight hold—can help ensure product integrity. Opaque tubing may also be used, and isolator lighting can be adjusted to filter out high-energy wavelengths, such as those in the blue and purple spectrum.
Once the drug product is filled into its final container—often amber glass for light-sensitive formulations—it becomes well-protected from further light exposure.
Sharp Sterile handled a project involving a product that was sensitive to both red and blue light, requiring protection under green light only. To ensure the product’s stability, the Sharp Sterile team covered cleanroom lights with sheeting and tubes to filter out red and blue wavelengths. Both the formulation and filling processes were conducted entirely under green light. Additionally, windows were covered to prevent external light from contaminating the controlled lighting conditions inside the cleanroom.
How to handle review and release of light-sensitive products
The next step in the process involves a visual inspection of the product. While there are no specific challenges when filling into amber vials, the use of amber containers can complicate visual inspections, as it becomes more difficult to detect particulates.
After inspection, light-sensitive products should be placed in packaging that will protect them from light exposure. For example, bulk vials can be stored in opaque boxes to prevent light from reaching the product. Ready-to-use containers—such as vials, syringes, or cartridges—should be returned to their nests or tubs. A transparent plastic cover, often with a foil layer, can be used to block light, or alternatively, an opaque cover can be applied for additional protection.
Protecting temperature-sensitive products
Temperature sensitivity is one of the most common challenges encountered by CDMOs. Many clients inquire whether their CDMO performs filtering and filling at room temperature. While it’s true that these processes often take place at room temperature, it’s important to note that there are strategies available to maintain the required temperature for sensitive products throughout the process. Even when operations occur at ambient temperature, the product can be kept chilled with appropriate measures in place.
Formulation of temperature-sensitive drug products
The formulation phase is the first part of the fill-finish process that may require modifications for temperature-sensitive products. Upon receipt, the drug product is unpackaged and inspected for any damage before being placed into the appropriate temperature-controlled unit, typically a freezer. For example, at Sharp Sterile, these shipments are scheduled, allowing the product to be inspected and moved into the appropriate temperature-controlled unit (TCU) within 10 minutes of receipt.
When preparing for formulation, the drug substance is removed directly from the freezer if it is in powder form. If the drug substance is a frozen solution, it is thawed in the appropriate temperature-controlled unit for a specified duration, as outlined in the batch record.
All materials are then brought into the formulation room, which is kept at room temperature, where the product is formulated according to the batch record. Jacketed mixing vessels can be used to regulate the temperature of the drug product during this process, ensuring it does not exceed the required temperature. Cooled water, maintained at 6–15°C, circulates through the jacketed vessels to control temperature.
Although the filtration process may not offer much opportunity for temperature control, it is typically brief enough that the exposure to room temperature during filtration does not significantly affect the product.
After formulation, the bulk drug product is commonly held overnight for filling the following day. However, if the product cannot withstand a hold, it must be immediately connected to the filling line.
How to handle filling for temperature-sensitive drug products
Temperature-sensitive drug products are typically filled from a water-jacketed vessel, which is cooled using a calibrated chiller unit. The chiller unit is positioned outside the isolator, adjacent to the bulk drug product solution. Water is circulated from the chiller unit into the vessel through a port at the bottom, filling the vessel from the bottom to the top. It then flows out through a port at the top of the vessel and returns to the chiller unit.
Once the product is filled, it is immediately transferred to a work-in-process cooler. Sharp Sterile’s coldest available work-in-process cooler is set to 2–8°C. The product will remain in this cooler until the visual inspection team is ready to conduct the inspection.
How to handle review and release of temperature-sensitive products
Temperature-sensitive drug products are typically inspected in a designated inspection area maintained at room temperature.
If there is concern about the duration of time a product spends in a 2–8°C cooler, clients may request expedited visual inspection so the product can be assessed as soon as possible. In more extreme cases, inspection can be performed off the line during the fill. In these instances, the product undergoes full visual inspection and Acceptable Quality Limit (AQL) testing before being immediately transferred to a frozen storage location.
When this approach is used, each tray of product is brought directly into the inspection suite immediately after it is filled. This allows visual inspection to occur concurrently with sterile filling operations.
Following inspection, the batch is moved to storage locations designated for finished product, where it remains until quality assurance (QA) review and final lot release. Once QA approval is received, the drug product is prepared for shipping. Packaging all chilled or frozen products are packaged with a device such as a TempTale allows the CDMO to continuously monitor and record temperature during transit.
This represents the complete fill-finish process for temperature-sensitive liquid fills. However, many clients choose lyophilization services to improve product stability and reduce the logistical challenges associated with cold-chain storage and distribution.
Lyophilization for temperature-sensitive products
The fill-finish process for lyophilized products is similar to that of liquid fills, with a few key differences.
For drug products that are highly unstable in solution, lyophilization—or freeze-drying—is often the preferred approach. These products typically arrive frozen and therefore follow the same formulation and filling procedures used for temperature-sensitive liquid products.
One notable difference during formulation is the addition of bulking agents, such as mannitol or sucrose. These excipients help produce a better-quality lyophilized cake. The appropriate formulation is determined during lyophilization cycle development.
Filling for lyophilized drug products proceeds much like it does for liquids, with the additional step of transferring filled vials into a lyophilizer.
Lyophilization provides strong protection for the drug product, so there is typically less urgency for expedited visual inspection. However, lyophilized products introduce different visual inspection criteria.
Inspectors must evaluate:
- Cake structure (proclivity)
- Cake color and any discoloration
- Evidence of collapse or shrinkage
- Presence of residual liquid in the vial
These factors are critical in determining whether a batch meets quality standards before it proceeds to release and distribution.
Protecting shear-sensitive products
Formulation of shear-sensitive products
Clients with shear-sensitive products are often concerned that mixing and pumping the solution can destroy or degrade their drug substance.
To address this, a CDMO may use magnetic stir bars and stir plates for gentle mixing during small-scale formulation. For larger-scale production, another choice is a Ross vertical blender. This equipment is specifically designed for shear-sensitive materials and uses a low-speed auger that rotates along the inner wall of a conical vessel, gently lifting and mixing the product with minimal agitation.
In some cases, shear sensitivity can also be reduced through formulation adjustments. Adding specific excipients can help protect the drug substance from shear-induced degradation. For instance, monoclonal antibodies are particularly prone to protein aggregation—a common issue linked to shear. Adjusting the physical or chemical composition of the formulation during development can significantly reduce the risk of this type of damage.
Fine-tuning the formulation early in development is key to ensuring a robust and scalable fill-finish process for shear-sensitive drug products.
Filling of shear-sensitive products
For shear-sensitive drug products, minimizing mechanical stress during filling is essential to preserving product integrity. Peristaltic pumps provide a gentle pumping action at low speeds to move the solution through the filter. Pressure filtration is another option for shear-sensitive products. Filling at a slow speed can reduce shear, but the best way to overcome any challenges with shear-sensitive products is to perform several fill studies in an engineering run to optimize the process and determine if changes can be made to reduce product degradation.
Visual inspection of shear-sensitive products
During visual inspection, operators typically swirl the container gently to help suspend and move any particulates, making them easier to detect. This motion is minimal and generally well-tolerated by most products. However, for especially shear-sensitive drug products, a client can make the choice that the product be gently inverted rather than swirled.
While this approach minimizes shear exposure, it can reduce the effectiveness of particulate detection. For this reason, inversion-only inspection should be reserved for the most extreme cases, where even minimal agitation poses a risk to product integrity.
Low-loss filling for shear sensitive products
Sharp Sterile offers a method of filling very low-volume batches (a liter of solution or less) that is excellent for shear-sensitive products. This method, SSM’s low-loss fill process, was designed to limit drug product loss.
Instead of using a pump, the system gently pressurizes the bulk drug product—usually around 1 PSI, or slightly higher for more viscous solutions. This light pressure provides the force necessary to move the product through the fill needle.
Dispensing is controlled by a time-operated actuator that is triggered by a foot pedal. When the actuator is triggered, it creates an opening allowing the product to be expelled through the fill needle, which then closes after the set duration. The volume of the product dispensed is controlled by increasing or decreasing the duration the actuator remains open, while keeping the pressure in the vessel constant.
Because this method avoids pumping entirely, it significantly reduces the risk of product degradation—making it an excellent choice for small-scale, shear-sensitive fills.
Conclusion
Protecting a drug product throughout the fill-finish process requires clear communication with the CMO and collaboration with a team that possesses the expertise and capabilities to accommodate product-specific sensitivities.
When a drug is in development, partnering with a CDMO that understands both formulation and manufacturing is essential. This ensures the drug product is not only scientifically viable but also manufacturable at scale.
At Sharp Sterile, product sensitivities can be identified early in development. Buffer composition, pH, and other formulation parameters are optimized to reduce sensitivity-related risks, and these formulations are tested on manufacturing equipment to confirm consistent, reliable performance.
With the right strategy and experience, even the most challenging drug products can be safely formulated, filled, and prepared for commercial success.



