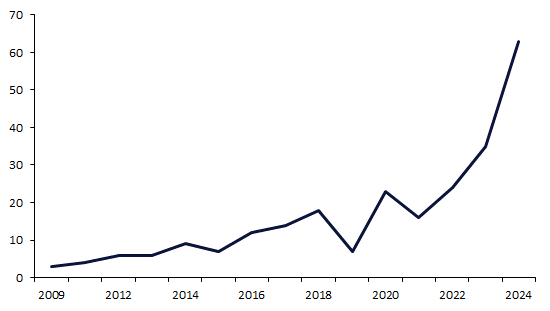
Antibody drug conjugates (ADCs) have gained significant momentum in recent years, due to their clinical successes in cancer treatment. Since 2019, the FDA has increasingly encouraged and accelerated ADC development through annual rises in review designation awarding. This trend reached new heights in 2024, when the FDA granted a record 63 designations to ADCs, nearly double the previous high of 35 in 2023.
ADCs utilise a linker to combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs, allowing them to selectively target and eliminate cancerous cells. This highly selective antibody-directed killing of cancer substantially improves upon the outcomes of conventional cancer therapies such as chemotherapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBetween 2009 and 2018, annual review designations awarded to ADCs increased steadily at a compound annual growth rate (CAGR) of 22%. Confidence in ADCs grew gradually over this period, as the science and manufacturing processes behind this technology were refined and validated. Despite a 2019 drop in review designation activity, the following five-year timeframe (2019 to 2024) represents an era of intense activity, as review designation awarding increased at a CAGR of 73.3%. In 2024, the FDA handed out a record of 63 designations, nearly double the previous peak of 35 in 2023 (Figure 1). The dramatic increase highlights the FDA’s growing intent to facilitate the market entry of these precision therapies.
Figure 1: Annual review designations by the FDA awarded to ADCs (2009-2014)
2019 represents a landmark year for ADCs, as three products received FDA approval: Daiichi Sankyo’s trastuzumab deruxtecan (Enhertu), Astellas’ enfortumab vedotin (Padcev), and Genentech’s polatuzumab vedotin (Polivy). This remains the highest number of ADC approvals in a single year. This historical year for ADCs, followed by the subsequent surges in review designation activity, suggests a deliberate regulatory shift towards fostering ADC products for cancer.
In 2024, 63 review designations were awarded to 35 individual ADCs. Comparatively, in 2019, seven designations were awarded to six drugs. This nine-fold increase in designation count, as well as six-fold increase in drug count, indicates that more companies are entering the ADC space, and that the FDA is actively supporting their development processes.
Out of all ADCs, Enhertu has collectively received the most review designations from the FDA, with 34. This is more than 2.5 times the total received by Takeda’s Adcetris, which ranks second. Some 92% of Enhertu’s review designations were awarded after the first FDA approval of this product, reflecting how its early clinical success prompted the FDA to accelerate its approval for alternative cancer types.
The precise nature of an ADC’s targeted approach allows for a more effective cancer treatment. Since the record year of ADC approvals in 2019, the FDA’s sustained increase in review designations underscores a regulatory commitment to advancing this technology and bringing these targeted therapies to patients more rapidly.
ADC content on Pharmaceutical Technology (Or Clinical Trials Arena) is supported by Syngene. Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.