Vetter Pharma, a German contract development and manufacturing organisation (CDMO), is developing a new clinical manufacturing facility in Des Plaines, Illinois, US.
The $285m brownfield redevelopment project will house the company’s main operations for North America, catering to aseptic filling, lyophilisation of injectable therapies, formulation, process development, and regulatory services.
Vetter Pharma obtained permission to build on the new site in April 2025 and initiated upstream and ground preparation work on the premises in the same month.
The ground-breaking ceremony held in June 2025 marked the start of construction work on the facility.
The site is expected to become fully operational by the end of 2029. It is expected to generate high-quality employment opportunities for the local community and contribute to economic growth.
The Des Plaines facility, along with Vetter Pharma’s clinical facility in Rankweil, Austria, will enable the company to address the growing development requirements for small-batch injectable formulations of innovative drug candidates that are in early clinical development.
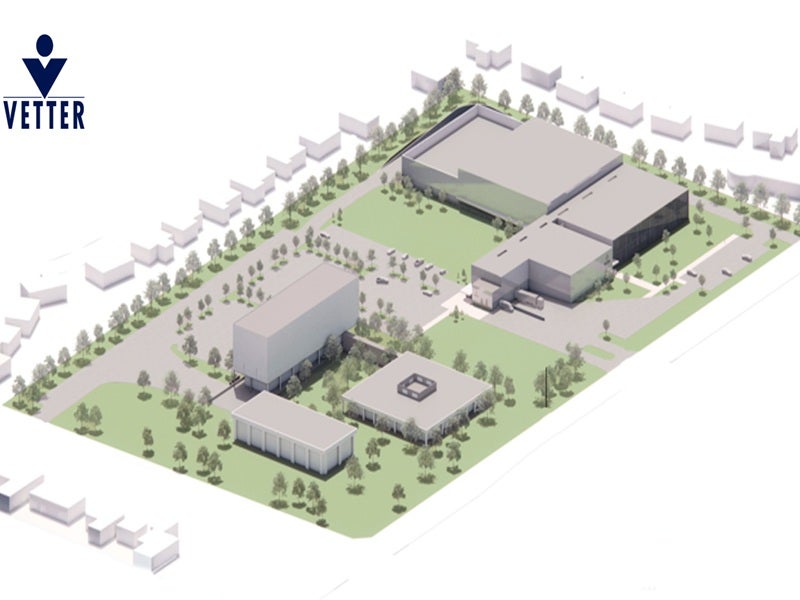
Location of Vetter Pharma’s new clinical manufacturing facility
Vetter Pharma’s clinical manufacturing site is under development within the Illinois Science and Technology Park, situated in Des Plaines in the greater Chicago area of Illinois, US.
Since 2009, the park has been instrumental in the launch of Vetter’s US manufacturing operations.
Nearly two years ago, the company decided to invest in a new clinical facility on its Des Plaines property, a move driven by the anticipated increase in customer demand and a strategy to expand capacity in response to future needs.
Following the completion of the new Des Plaines clinical facility, Vetter Pharma plans to relocate its existing clinical operations from Skokie, Illinois, to the new facility. The Skokie site is said to have played a vital role in establishing the company’s US market presence.
Vetter Pharma clinical manufacturing facility details
Vetter Pharma’s planned 160,000ft² clinical production facility will focus on aseptic manufacturing.
It will include a main production area and feature increased capacity for the production of small batches of novel active ingredients, with designated spaces for material preparation, as well as compounding.
The facility will also include necessary support infrastructure such as storage and office spaces to create an integrated operational framework.
Construction plans include the erection of new buildings for clinical batch filling and associated services, while maintaining the current buildings in the area.
The 860,000ft² property also allows for potential future growth based on market conditions, customer requirements, and parallel investment initiatives by Vetter Pharma in Germany and Austria.
Contractors involved
The project involves several contractors, including CRB, an engineering, architecture, construction and consulting solutions provider, and Evans General Contractors, a commercial construction company.
CRB is serving as the engineer of record, process architect, and lab planner for the project.
Other businesses involved in the project include Mackie Consultants, Legat Architects, and Thornton Tomasetti, a science and engineering consulting company.
Marketing commentary on Vetter Pharma
Vetter is an independent, family-run company founded in 1950 in Ravensburg, Germany.
The company offers a comprehensive suite of services, ranging from the development of drug products to clinical and commercial filling, as well as packaging services for an array of delivery systems, including cartridges, syringes, and vials.
Vetter operates 21 aseptic filling lines across manufacturing facilities in the US and Europe, with production sites in Germany, Austria, and the US, and sales locations in the Asia-Pacific markets of Japan, China, South Korea, and Singapore.
Last year, Vetter Pharma announced plans for further investments in its commercial business in south-west Germany, which borders France and Luxembourg, to support the growth and expansion of capacity in Ravensburg and Langenargen, which are currently in progress.