Researchers at the Massachusetts Institute of Technology (MIT) in the US have developed a new single-injection vaccine that can carry several doses in nanoparticles.
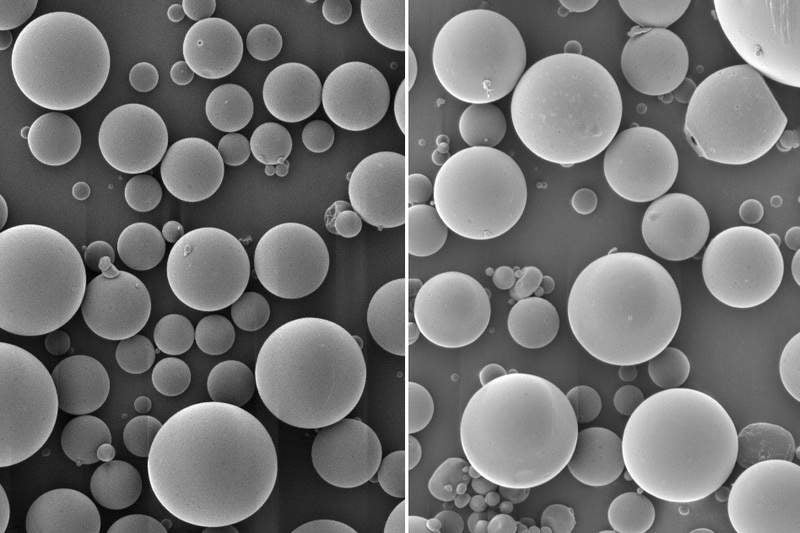
Intended to facilitate easy immunisation, the new product encapsulates inactivated polio vaccine in a biodegradable polymer called PLGA, which can degrade after a certain timeframe.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
These polymer microspheres allow the release of the vaccine in two different bursts. The researchers used positively charged polymers in order to prevent the effect of byproducts, glycolic acid and lactic acid on the virus.
MIT former postdoc Stephany Tzeng said: “There’s always a little bit of vaccine that’s left on the surface or very close to the surface of the particle, and as soon as we put it in the body, whatever is at the surface can just diffuse away. That’s the initial burst.
“Then the particles sit at the injection site and over time, as the polymer degrades, they release the vaccine in bursts at defined time points, based on the degradation rate of the polymer.”
In order to test the single-injection vaccine, the MIT team designed nanoparticles to deliver an initial burst virus at the time of injection and another after approximately 25 days later, and injected them into rats.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataUpon analysis at the Centers for Disease Control, blood samples from the immunised rats revealed that antibody response with the new vaccine was as strong as or stronger when compared to two injections of the Salk polio vaccine.
The researchers said that they could also design and combine particles that can release one dose at injection and a second one after two months, with those releasing vaccine at injection and one month later.
These polymers are already approved by the US Food and Drug Administration (FDA) for in-human use, and the team is planning to evaluate the vaccines in clinical trials.
The researchers are also in the process of employing the technique to devise stable single-injection vaccines for other viruses, including Ebola and HIV.




