Biogen/Eisai‘s newly approved drug, Leqembi (lecanemab), gained FDA approval in January 2023 for the treatment of Alzheimer’s disease. The breakthrough drug is predicted to be a blockbuster, generating total forecast sales of $12.9bn between 2023 and 2028. Leqembi is currently pending approval in the EU and Japan. If approved in these regions, it would be established as the top-selling drug for Alzheimer’s disease in the coming years.
Leqembi is a monoclonal antibody that targets amyloid beta A4 protein and acts by selectively binding and eliminating amyloid beta plaques, thought to be clinically significant for the treatment of Alzheimer’s disease. The FDA’s decision on Leqembi was based on data from a Phase II clinical trial named Athena AD. The results of the trial showed that the treatment led to a significant reduction in the number of amyloid beta plaques in the brain, and demonstrated a statistically significant reduction in cognitive decline in patients treated with Leqembi compared to those who received a placebo.
© GlobalData Plc.
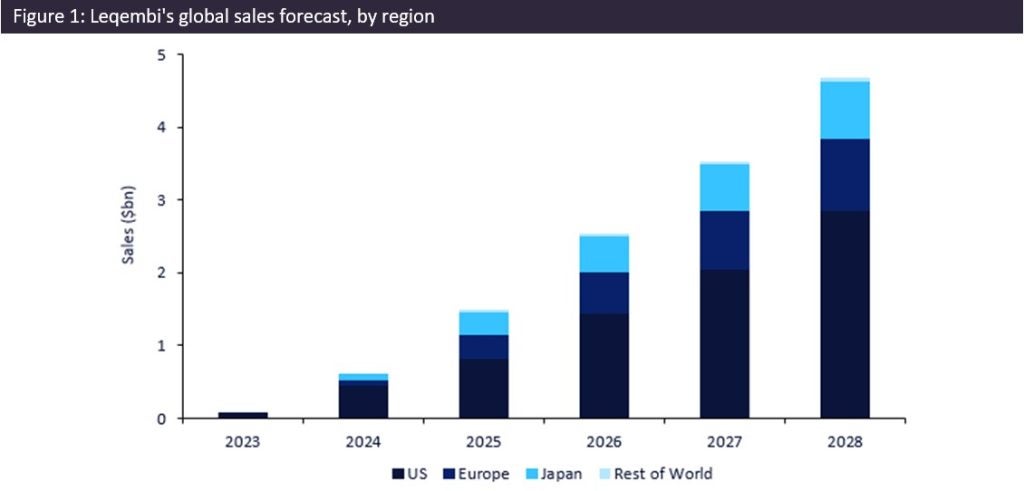
Leqembi is forecast to generate total sales of $12.9bn worldwide between 2023 and 2028. This projection surpasses that of Aricept, the second-largest approved Alzheimer’s drug, with forecast total sales of $762m between 2023 and 2028. This would make Leqembi’s forecast sales 17 times greater than those of Aricept.
Leqembi’s largest market is expected to be the US, contributing $7.7bn of 2023–28 forecast sales. The anticipated regulatory approvals of Leqembi in Europe and Japan in October 2023 are expected to contribute to revenue growth. Sales in these regions are projected to surge at a compound annual growth rate (CAGR) of 77% until 2028, accounting for 39% of the drug’s 2023–28 total revenue share. However, the US market remains crucial in driving the drug’s commercial success, with forecast sales projected to increase at a CAGR of 106% until 2028, 60% of the drug’s total revenue share.
Leqembi faces competition from Eli Lilly’s donanemab, a similar monoclonal antibody targeting amyloid beta plaques, with forecast total sales of $8.1bn by 2028. However, in January 2023, the FDA rejected Eli Lilly’s bid for accelerated approval of donanemab, leaving Leqembi as the leading drug in this space.
Biogen/Eisai’s newly approved drug, Leqembi, offers new hope to patients suffering from Alzheimer’s disease. The forecast total sales of $12.9bn by 2028 underscores the significant impact Leqembi is expected to have on the market. With the rejection of donanemab’s bid for accelerated approval, Leqembi is in a favourable position, expected to emerge as one of the best-selling drugs for Alzheimer’s disease in the coming years.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData



